Does ammonium nitrate occur naturally?
OVERVIEW. Ammonium nitrate (uh-MOH-ni-um NYE-trate) is a white crystalline substance first made artificially in 1659 by the German chemist Johann Rudolf Glauber (1604–1670). The compound does not occur in nature because it is so soluble that it is washed out of the soil by rain and surface water.
How do you make ammonium nitrate from scratch?
2:197:06Easiest way to make ammonium nitrate - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe chemical reaction that's going on here is between nitric acid hno3. Plus and h 4o h ammonia. OrMoreThe chemical reaction that's going on here is between nitric acid hno3. Plus and h 4o h ammonia. Or as it's commonly known in water ammonium hydroxide.
What is ammonium nitrate fertilizer made of?
ammonium nitrate, (NH4NO3), a salt of ammonia and nitric acid, used widely in fertilizers and explosives. The commercial grade contains about 33.5 percent nitrogen, all of which is in forms utilizable by plants; it is the most common nitrogenous component of artificial fertilizers.
Why ammonium nitrate is so explosive?
At high enough temperatures, however, ammonium nitrate can violently decompose on its own. This process creates gases including nitrogen oxides and water vapour. It is this rapid release of gases that causes an explosion.
Can you legally buy ammonium nitrate?
Some countries are phasing out or banning the compound because of its potential use in improvised explosive devices, or IEDs. But in the US, you can buy ammonium nitrate on Amazon, at gardening stores, or from agricultural suppliers.
Who produces ammonium nitrate?
Russian Federation is the top country by ammonium nitrate production in the world. As of 2020, ammonium nitrate production in Russian Federation was 11.1 million tonnes that accounts for 67.11% of the world's ammonium nitrate production.
What two chemicals will explode when mixed?
Peroxides (inorganic), when mixed with combustible materials, barium, sodium, and potassium, form explosives that ignite easily. Phosphorus (P), both red and white, forms explosive mixtures with oxidizing agents. White (also called yellow) P should be stored under water, in glass, because it is pyrophoric.
Is ammonium nitrate mined or manufactured?
Ammonium nitrate was mined there until the Haber–Bosch process made it possible to synthesize nitrates from atmospheric nitrogen, thus rendering nitrate mining obsolete.
What is a substitute for ammonium nitrate?
Potassium nitrate is an excellent substitute for ammonium nitrate.
How can I make nitric acid at home?
0:397:05Make Nitric Acid - The Complete Guide - YouTubeYouTubeStart of suggested clipEnd of suggested clipAll right add around 50 milliliters of water to your nitrate. And try to dissolve as much of it asMoreAll right add around 50 milliliters of water to your nitrate. And try to dissolve as much of it as you can then carefully add 100 milliliters of hydrochloric acid.
How do you make ammonia at home?
5:3510:02Make Concentrated Ammonia - YouTubeYouTubeStart of suggested clipEnd of suggested clipThis is done slowly as the heat produced can actually boil the solution. Well that's happening in aMoreThis is done slowly as the heat produced can actually boil the solution. Well that's happening in a separate container we mix 240 grams of ammonium nitrate with 160 milliliters of water.
How do you get ammonium nitrate from cold packs?
0:433:41Ammonium Nitrate from Cold Packs - YouTubeYouTubeStart of suggested clipEnd of suggested clipSalts. So all that's in here aside from the ammonium nitrate is some water and that's in a littleMoreSalts. So all that's in here aside from the ammonium nitrate is some water and that's in a little pouch and it'll be super easy to remove.
What is ammonium nitrate used for?
Used to make fertilizers and explosives, and as a nutrient in antibiotic and yeast growth. Ammonium nitrate is the nitric acid ammonium salt. It ha...
Is ammonium nitrate a base or acid?
Ammonium nitrate is not acid but a salt but the solution is acidic since it is a salt with a weak base (ammonium hydroxide) and a heavy nitric acid.
What happens when you mix water and ammonium nitrate?
It feels cold when ammonium nitrate is dissolved in water which indicates an endothermic reaction. In an endothermic reaction, the ammonium nitrate...
What can you do with ammonium nitrate?
Ammonium nitrate is a chemical compound used as a fertilizer in agriculture, for producing pyrotechnics, as an ingredient in cold packs, and for sc...
Is ammonium nitrate a good fertilizer?
Ammonium nitrate fertilizer is the compound’s most common application but it also has a very volatile quality, making it useful in some industries....
What is Ammonium Nitrate?
Ammonium nitrate is an ionic salt made up of the ammonium cation (NH4)+ and the nitrate anion (NO3)–. The chemical formula of this compound is NH4NO3.
What is the structure of ammonium nitrate?
Structure of Ammonium Nitrate. This compound features an ionic bond between an ammonium ion and a nitrate ion. The structure of an NH 4 NO 3 molecule is illustrated below. The pi electrons in the nitrate ion are delocalized due to resonance. The net charge on this ion is -1 (since the nitrogen atom holds a charge of +1 and each oxygen atom holds ...
Why does ammonium nitrate feel cold?
It feels cold when ammonium nitrate is dissolved in water which indicates an endothermic reaction. In an endothermic reaction, the ammonium nitrate dissolves in water, a chemical reaction that absorbs heat rather than releases it. Ammonium nitrate consists of tightly packed, ionic bonds.
How to make NH4NO3?
NH 4 NO 3 can be prepared from the acid-base reaction between nitric acid and ammonia, described by the following chemical equation: NH3 + HNO3 → NH4NO3. This reaction is highly exothermic and proceeds violently. Ammonium nitrate is also known for its oxidizing powers.
Why is ammonium nitrate being phased out?
Despite its numerous applications, the use of this compound is being slowly phased out by the governments of many countries due to its scope for misuse. Ammonium nitrate was one of the key ingredients of the explosives that were used in the 2011 Delhi bombings and the 2013 blasts in Hyderabad.
What is the name of the explosive that is mixed with fuel oil?
When mixed with fuel oil, it forms an explosive known as ANFO, which is one of the most popular industrial explosives.
Is ammonium nitrate an explosive?
When exploded, this compound yields N 2, O 2, and water as the by products. Despite being a component of many explosives, ammonium nitrate is not an explosive by itself. It must be mixed with a primary explosive such as an azide to form an explosive mixture.
What is the chemical binding of ammonium nitrate?
Ammoinium nitrate chemical bindings and formula. Ammonium nitrate was said to be developed Germans which they used as fertilizers instead of Chilean Nitrates since it is a lot cheaper. Commercially, it is prepared by mixing nitric acid and ammonia salt. The reaction from the two substances combined will form Ammonium Nitrate.
Why is ammonium nitrate used in agriculture?
It is actually sold in the form of pellets that are coated with clay. The reason why it is very popular in agriculture is because of the high nitrogen amount in this compound. Nitrogen is a very important plant nutrient that assists in the growth and metabolic processes that the plant undergoes.
What is anfo made of?
ANFO is composed of 94 percent ammonium nitrate and 6 percent fuel oil. The ammonium nitrate will serve as the oxidizing agent for the fuel. Another interesting fact about this compound is that it is actually hygroscopic. A hygroscopic substance is something that can easily collect water molecules from the environment where it is placed.
What is the name of the compound that is made of nitric acid and salt of ammonia?
Interesting Facts about Ammonium Nitrate. Ammonium nitrate is a chemical compound with the formula NH4NO3. It is composed of nitric acid and salt of ammonia. In room temperature, ammonium nitrate appears in a white crystalline form and it is also colorless.
Why is ammonium nitrate paired with TNT?
It is a strong oxidizing agent. This means that it can actually remove certain electrons from other reactants when subjected to a redox chemical reaction. This is the reason why ammonium nitrates are paired and added in combustibles like TNT and others.
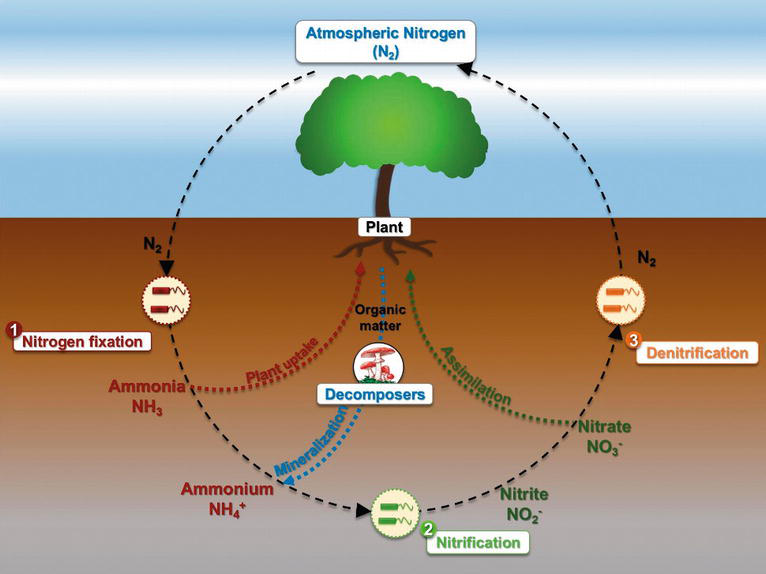
Why is nitrogen important for plants?
Nitrogen is a very important plant nutrient that assists in the growth and metabolic processes that the plant undergoes . Agriculturists love using ammonium nitrate since it is a cheap alternative to expensive fertilizers. It can also yield rapid growth and may increase the fruit production capacity of a plant.
Does ammonium nitrate affect the quality of green leafy vegetables?
It can also yield rapid growth and may increase the fruit production capacity of a plant. It may also affect the quality of green leafy vegetables since the nitrogen which is used by the plants is actually very helpful in the process of photosynthesis. Another famous use of ammonium nitrate is as an additive in explosives.
Why is ammonium nitrate used in dynamite?
Because solid ammonium nitrate can undergo explosive decomposition when heated in a confined space, government regulations have been imposed on its shipment and storage . After the straight dynamites and gelatins, the next important advance in dynamite was the substitution of ammonium nitrate for part of the...
What is the most common nitrogenous component of artificial fertilizers?
Ammonium nitrate, a salt of ammonia and nitric acid, used widely in fertilizers and explosives. The commercial grade contains about 33.5 percent nitrogen, all of which is in forms utilizable by plants; it is the most common nitrogenous component of artificial fertilizers.
What is NH4NO3 used for?
ammonium nitrate, (NH4NO3), a saltof ammoniaand nitric acid, used widely in fertilizers and explosives. The commercial grade contains about 33.5 percent nitrogen, all of which is in forms utilizable by plants; it is the most common nitrogenous component of artificial fertilizers. Ammonium nitratealso is employed to modify the detonation rate of other explosives, such as nitroglycerinin the so-called ammonia dynamites, or as an oxidizing agent in the ammonals, which are mixtures of ammonium nitrate and powdered aluminum.
What is the most common nitrogen in fertilizer?
The commercial grade contains about 33.5 percent nitrogen, all of which is in forms utilizable by plants; it is the most common nitrogenous component of artificial fertilizers. Ammonium nitrate also is employed to modify the detonation rate of other explosives, such as nitroglycerin in the so-called ammonia dynamites, ...
Is ammonium nitrate a crystalline substance?
Ammonium nitrate is a colourless crystalline substance (melting point 169.6 °C [337.3 °F]). It is highly soluble in water; heating of the water solution decomposes the salt to nitrous oxide(laughing gas). Because solid ammonium nitrate can undergo explosive decomposition when heated in a confined space, government regulations have been imposed on its shipment and storage.
How is ammonium nitrate produced?
Ammonium nitrate (NH4NO3) is produced by neutralizing nitric acid (HNO3) with ammonia (NH3) . ... All ammonium nitrate plants produce an aqueous ammonium nitrate solution through the reaction of ammonia and nitric acid in a neutralizer.
What is ammonium nitrate?
CAMEO Chemicals. Ammonium Nitrate Emulsion, Suspension, or Gel is ammonium nitrate suspended in a liquid. The material itself does not readily burn but will readily do so if contaminated by combustible material.
What is the Ammonium Nitrate Security Program?
Ammonium Nitrate Security Program; Proposed Rule: This proposed rule would implement anti-terrorism measures to better secure the homeland. The Department of Homeland Security would regulate the sale and transfer of ammonium nitrate pursuant to section 563 of the Fiscal Year 2008 Department of Homeland Security Appropriations Act with the purpose of preventing the use of ammonium nitrate in an act of terrorism. This proposed rule seeks comment on both proposed text for such a regulation and on several practical and legal issues integral to the development of an Ammonium Nitrate Security Program.
How to prevent ammonium nitrate explosion?
Actions that may help to prevent explosions include: (1) Avoid heating ammonium nitrate in a confined space (e.g., processes involving ammonium nitrate should be designed to avoid this possibility). (2) Avoid localized heating of ammonium nitrate, potentially leading to development of high temperature areas. (3) Ensure that ammonium nitrate is not exposed to strong shock waves from explosives. (4) Avoid contamination of ammonium nitrate with combustible materials or organic substances such as oils and waxes. (5) Avoid contamination of ammonium nitrate with inorganic materials that may contribute to its sensitivity to explosion, including chlorides and some metals, such as chromium, copper, cobalt, and nickel. (6) Maintain the pH of ammonium nitrate solutions within the safe operating range of the process. In particular, avoid low pH (acidic) conditions.
How much ammonium nitrate is in a solid?
Approximately 60 percent of the ammonium nitrate produced in the U. S. is sold as a solid product. To produce a solid product, the ammonium nitrate solution is concentrated in an evaporator or concentrator. The resulting "melt" contains about 95 to 99.8 percent ammonium nitrate at approximately 149 °C (300 °F).
How many people are exposed to ammonium nitrate?
According to the 2006 TSCA Inventory Update Reporting data, the number of persons reasonably likely to be exposed in the industrial manufacturing, processing, and use of ammonium nitrate is 1000 or greater; the data may be greatly underestimated (1).
How much nitrogen is lost from fertilizer?
The total immediate loss of nitrous oxide - nitrogen after application of mineral fertilizer is estimated to be 0.004-1.2 teragram/yr.
What is Ammonium Nitrate?
Nitrogen comes in many forms. This major plant nutrient can be taken in by plants through the roots or from the stoma in the leaves and stems. Additional sources of nitrogen are often added to soil and plants in areas without sufficient natural sources of nitrogen.
How is ammonium nitrate fertilizer made?
Ammonium nitrate fertilizer is a simple compound to make. It is created when ammonia gas reacts with nitric acid. The chemical reaction produces a concentrated form of ammonium nitrate, which produces prodigious amounts of heat. As a fertilizer, the compound is applied as granules and fused with ammonium sulfate to minimize the volatile nature of the compound. Anti-caking agents are also added to the fertilizer.
What are the sources of nitrogen in soil?
One of the first solid nitrogen sources produced in a large scale capacity is ammonium nitrate.
Is ammonium nitrate explosive?
In addition to its usefulness as a fertilizer, ammonium nitrate is also employed in certain industrial and construction settings. The chemical compound is explosive and useful in mining, demolition activities, and quarry work.
Can ammonium nitrate be used in food preservation?
In most cases, the compound is very stable and can only become explosive in certain conditions. Food preservation is another area that is using ammonium nitrate. The compound makes an excellent cold pack when one bag of water and one bag of the compound are united.
Is ammonium nitrate a stable compound?
Ammonium nitrate in gardens is made stable with other compounds. The fertilizer is an almost instantly useable form of nitrogen due to its porosity and solubility. It provides nitrogen from both ammonia and nitrate. The standard method of application is by broadcast spreading the granules.