What are some examples of ionic bonds?
Some ionic bond examples include:
- NaCl: sodium chloride
- NaBr: sodium bromide
- NaF: sodium fluoride
- NaI: sodium iodide
- KF: potassium fluoride
- KCl: potassium chloride
- KI: potassium iodide
- KBr: potassium bromide
- LiI: lithium iodide
- Li2O: lithium oxide
What are 5 examples of ionic compounds?
Ionic bond examples include:
- LiF - Lithium Fluoride
- LiCl - Lithium Chloride
- LiBr - Lithium Bromide
- LiI - Lithium Iodide
- NaF - Sodium Fluoride
- NaCl - Sodium Chloride
- NaBr - Sodium Bromide
- NaI - Sodium Iodide
- KF - Potassium Fluoride
- KCl - Potassium Chloride
Which elements form ionic bonds?
- All transition metals and rare earth metals act as positives in ionic bonding.
- Hydrogen can be involved in ionic bonding. It will act as a nonmetal with anegative one charge. It is named hydride.
- There are more complex ionic bonding situations which will remain for later. For example, the bond between NH 4+ and Cl¯ in ammonium chloride is an ionic bond.
What are common ionic bonds?
Many common ionic compounds contain polyatomic ions, such as these ionic compound examples:
- Antacids - are used to combat heartburn and contain magnesium hydroxide, Mg (OH) 2, an ionic compound.
- Chalk - blackboard chalk contains calcium carbonate, CaCO 3.
- Baking soda - this is the common name for sodium bicarbonate, NaHCO 3. ...
- A common fertilizer, ammonium nitrate, NH 4 NO 3 - this is composed of two polyatomic ions.

What are 3 examples of an ionic bond?
Examples of Ionic Bonds Some ionic bond examples include: NaCl: sodium chloride. NaBr: sodium bromide. NaF: sodium fluoride.
How is ionic bonding formed?
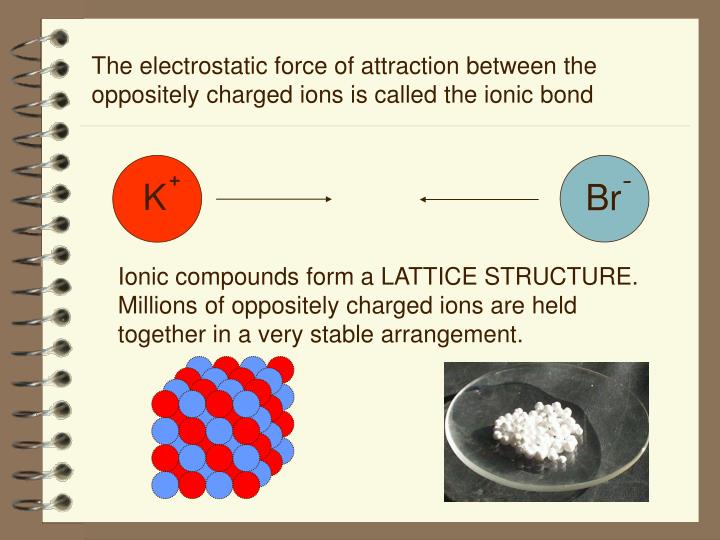
ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
What is one example of an ionic bond?
An example of an ionic bond is the bond in sodium chloride, which is salt. Sodium's valence electron is transferred to the outer electron shell of chloride. Molecules with ionic bonds form ionic compounds.
What is ionic bond Simple?
One ionic bond definition is that atoms are held together by charge. Plus sticking to minus. Charged atoms are called ions, and they can clump like a pile of magnets locked together. The charged atoms clump together in a very specific geometric pattern that we often call a crystal structure.
What is most likely to form an ionic bond?
An ionic bond is most likely to form between metal and nonmetal elements. An element can be classified as a metal if it's found on the left side of the periodic table, while nonmetals are located on the right side of the periodic table.
What is an ionic bond give two examples?
An ionic bond is formed as a result of the electrostatic attraction between the positive and negative ions formed by the transfer of electrons from one atom/group to another. Examples: NaCl,KBr,etc.
What are the 5 example of ionic compound?
Examples of ionic compounds in everyday life include table salt, baking soda, lye, Epsom salt, and bleach.
What are the example of ionic?
Table salt is an example of an ionic compound. Sodium and chlorine ions come together to form sodium chloride, or NaCl. The sodium atom in this compound loses an electron to become Na+, while the chlorine atom gains an electron to become Cl-.
Which best describes how an ionic bond forms?
Which statement best describes how an ionic bond forms? The transfer of electrons forms strong bonds between ions.
How do ions form ionic bonds quizlet?
How do ions form ionic bonds? Two atoms come together to share their electrons. Ions of opposite electrical charges are attracted to each other to balance the charges. One atom swaps all of its negative electrons for all of the other atom's positive protons.
Why do ions form ionic bonding?
When metals and non-metals react, the metals lose electrons by transferring them to the non-metals, which gain them. Consequently, ions are formed, which instantly attract each other—ionic bonding.
How ionic and covalent bonds are formed?
Ionic compounds are (usually) formed when a metal reacts with a nonmetal (or a polyatomic ion). Covalent compounds are formed when two nonmetals react with each other. Since hydrogen is a nonmetal, binary compounds containing hydrogen are also usually covalent compounds.
What is an ionic bond vs covalent?
An ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. Charged chemical species form when neutral atoms,...
What is ionic bond explain with example?
An ionic bond is a bond between two oppositely charged chemical species which can be singular atoms or a group of atoms. Of the two charged species...
What are 3 examples of an ionic bond?
Three examples of an ionic bond are: (1) NaCl (sodium chloride or commonly called table salt) produced from cation Na+ and anion Cl-. (2) KMnO4...
Which of the following is example of ionic bond?
(1) Water - which is made from two atoms of hydrogen bonded with one atom of oxygen (2) Sodium Bicarbonate - which is made from sodium ion bonded...
Frame the formula for the compound formed when magnesium and fluorine combine?
Mg forms a divalent ion of Mg2plus. To obey the octet rule, Mg gets rid of the two valence electrons present in its valence shell. Fluorine has sev...
Which elements most often form ionic bonds?
Ionic bonds most commonly form between metals and non-metals. There is a large electronegativity difference between metals and non-metals. If the d...
Does oxygen form ionic bonds?
Oxygen does not contain ionic bonds. There is a mutual sharing of electrons between two oxygen atoms which results in the formation of a covalent b...
Is water covalent or ionic?
Water is a polar covalent molecule, which easily dissolves most of the substances. As oxygen is more electronegative than the hydrogen atom, the sh...
What are some examples of ionic bonds?
Ionic bond examples include: LiF - Lithium Fluoride. LiCl - Lithium Chloride.
What is an ionic bond?
Ionic bonds are atomic bonds created by the attraction of two differently charged ions. The bond is typically between a metal and a non-metal. The structure of the bond is rigid, strong and often crystalline and solid. Ionic bonds also melt at high temperatures. Dissolved in water, ionic bonds are aqueous, that is, they can conduct.
What is an ionic bond?
See Article History. Alternative Titles: electrovalency, electrovalent bond, heteropolar bond, polar bond. Ionic bond, also called electro valent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence ...
When do ionic and covalent bonds form?
Ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent bonds form when the electronegativities are similar. Compare covalent bond. The Editors of Encyclopaedia Britannica This article was most recently revised and updated by Adam Augustyn, Managing Editor, Reference Content.
What is the ionic bond in sodium chloride?
ionic bond: sodium chloride, or table salt. Ionic bonding in sodium chloride. An atom of sodium (Na) donates one of its electrons to an atom of chlorine (Cl) in a chemical reaction, and the resulting positive ion (Na +) and negative ion (Cl −) form a stable ionic compound ...
How many electrons does sodium have?
The sodium atom has a single electron in its outermost shell, while chlorine needs one electron... Ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals and the alkali and alkaline-earth metals.
Which atom has a single electron in its outermost shell?
Sodium chloride exhibits ionic bond ing. The sodium atom has a single electron in its outermost shell, while chlorine needs...
Is an ionic crystal hard or nonvolatile?
The magnitude of the electrostatic forces in ionic crystals is considerable. Accordingly, these substances tend to be hard and nonvolatile.
What are the ionic and covalent bonds?
Covalent Bonds. The electrons that are found in the outermost shell (valence shell) of an atom are known as the valence electrons and are responsible for forming bonds between two atoms. These bonds can be between two or more atoms of the same element or two or more atoms of different elements.
Which type of bond is stronger, covalent or ionic?
Though both kinds of bonds result in more stable molecules of lower energy, ionic bonds are generally stronger than covalent bonds and break apart completely into their respective ions in polar liquids.
How does an anion form?
In order for an anion to form, the atom has to gain one or more electron in its outer shell. The addition of electrons disrupts the neutral charge, and gives the atom a net negative charge.
How do covalent bonds form?
Covalent bonds are formed when two neutral atoms come together to share one or more of their valence electrons. The electron spends time between both atoms, thereby forming a covalent bond.
Where does a cation form?
The cation is usually formed from the atom of a metal element and the anion is formed from the atom of a nonmetal element.
Where are electrons found in an atom?
The electrons are found in the shells of an atom, surrounding its nucleus. An atom. The atomic number of an element signifies the number of protons, neutrons, and electrons that the atom contains. All atoms are neutral, which means that they have equal numbers of protons and electrons.
Why are ions not neutral?
Ions are not neutral because the number of protons does not equal the number of electrons. Two or more atoms bonded to each other can also carry an overall positive or negative charge and these are called polyatomic ions.
How are the constituent ions of an ionic compound held together?
In any crystal, the constituent ions of the ionic compound are held together by electrostatic forces of attraction. The stronger the forces of attraction, the higher is the lattice energy and the more stable is the compound. This electrostatic force of attraction is determined by Coulomb’s Law.
Why are ionic compounds brittle?
Ionic compounds are brittle – When an external force is applied to the crystals of an ionic compound, it shatters into pieces. This happens because, in the crystals of sodium chloride, the Na + ions and Cl – ions are lined up against each other in a lattice with a strong electrostatic force of attraction.
What is the ionic bond of table salt?
We are all quite familiar with table salt that we use to add flavour to food. We also know that chemically, table salt is sodium chloride in which sodium and chloride ions are bonded together by ionic bonds. Various factors affect the formation of these ions and consequently ionic bonding, which ultimately gives rise to ionic compounds. The factors are –
What is the term for the transfer of electrons from an electronegative element to an atom of an electronegative element?
The chemical bond that is formed between 2 atoms through the transfer of one or more electrons from the electropositive or metallic element to the atom of an electronegative or non-metallic element is called an ionic or electrovalent bond .
Why should ions combine?
Ions should combine in a way that the charges of the ions must balance out and the overall ionic compound is neutral.
What are compounds made of cations and anions called?
Compounds composed of cations and anions are called ionic compounds:
What is the ionization energy of sodium?
Consider the formation of sodium ion (Na +) from sodium atom. The ionization energy of sodium is about 500kJ / mol, which is quite low. It can easily lose electrons and get converted into a sodium ion. This ion can further take part in ionic bond formation with other anions such as Cl –, Br –.
Sodium Chloride NaCl
Sodium Chloride, Sodium atom has 1 valence electron and Chlorine atom has 7 valence electrons. Chlorine atom need one electron to complete its octet. Na atom loses its electron and acquires a positive charge while Chlorine atom gains an electron and acquires a negative charge. Hence Na and Cl form an ionic bond.
Sodium Bromide NaBr
In Sodium Bromide, the Sodium atom has one electron in its valence shell and the Bromine atom has 7 electrons. Na loses one electron which is gained by Br to complete its octet. Ionic compound NaBr is formed.
Sodium Fluoride NaF
In Sodium Fluoride NaF, to complete octet state Fluorine atom need only 1 electron, which is given by the sodium atom. Na acquires positive [Na] + and F acquire negative charge [F] –, forming an ionic bond.
Potassium Chloride KCl
In Potassium Chloride KCl, the Potassium atom has one electron in its valence shell and the Chlorine atom has seven electrons. Chlorine atom need one electron to complete its octet state. K loses its electron and becomes positively charged by gaining this electron Cl becomes negatively charged. The Formation Ionic bond takes place between K and Cl.
Potassium Iodide KI
In Potassium Iodide KI, the Iodine atom has seven valence electrons in its valence shell to get its octet state complete, it requires one electron. Potassium loses one electron, acquires a positive charge while iodine takes this electron and acquires a negative charge forming an ionic bond.
Potassium Bromide KBr
In Potassium Bromide KBr, Potassium has 1 electron in its valence shell whereas Bromine has seven electrons. Hence K loses its electron becomes K + and Br gains this electron becomes Br –. The ionic bond formed between K and Br.
Potassium Fluoride KF
In Potassium Fluoride KF, the Fluorine atom has seven electrons and the Potassium atom has one electron in its valence shell. To get a stable configuration Fluorine needed one electron. Potassium transfers its valence electron to Fluorine forming an ionic bond.
What type of bond is formed when two opposite charges are attracted to each other?
The two opposite charges will be attracted towards each other and form an ionic bond . Many compounds in the world are formed by ionic bonds and are therefore ionic compounds. Different types of salts are ionic compounds.
What type of bond is formed when a metal transfer electrons to a nonmetal?
Ionic Bonds. An ionic bond is a type of chemical bond that has formed as a result of the complete transfer of valence electrons from one molecule to another. Metals will transfer their valence electrons to non-metals forming a positively-charged ions.
Why are ionic compounds called ionic compounds?
Ionic bonds form between two atoms that have different electronegativity values. Because the ability to attract electrons is so different between the atoms, it's like one atom donates its electron to the other atom in the chemical bond.
What is the name of the ionic compound that is written before the anion?
MgO: magnesium oxide. Note that ionic compounds are named with the cation or positively-charged atom written before the anion or negatively-charged atom. In other words, the element symbol for the metal is written before the symbol for the nonmetal.