Formation of copper (I) oxide is the basis of the Fehling's test and Benedict's test for reducing sugars. These sugars reduce an alkaline solution of a copper (II) salt, giving a bright red precipitate of Cu 2 O. It forms on silver -plated copper parts exposed to moisture when the silver layer is porous or damaged.
What is Cu2O made of?
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper, the other being or copper(II) oxide or cupric oxide (CuO). This red-coloured solid is a component of some antifouling paints.
How do you synthesize Cu2O?
Cuprous oxide (Cu2O) nanoparticles are synthesized by a wet chemical reduction method using copper sulfate as a precursor. X-ray diffraction pattern reveals the structure of Cu2O nanoparticles is single phase cubic with an average crystallite size of 6.8 nm.
How is CuO formed?
It can be formed by heating copper in air at around 300–800°C: 2 Cu + O2 → 2 CuO.
Does CuO and Cu2O have the same properties?
Cuprous oxide is Cu2O while cupric oxide is CuO. The key difference between cuprous oxide and cupric oxide is that cuprous oxide has a dark red colour whereas cupric oxide has a black colour. The IUPAC name of cuprous oxide is copper(I) oxide while the IUPAC name of cupric oxide is copper(II) oxide.
What is the difference between Cu2O and CuO?
Cu2O is obtained by oxidation of copper metal or reduction of copper(II) solutions with sulfur oxide, whereas CuO is obtained by pyrometallurgical processes used to extract copper from ores. Many of the wood preservatives are made of copper. It is also used as a pigment to create different glazes.
What happens when you heat Cu2O?
Solution. When a mixture of Cu2O and Cu2S is heated, they react together to give copper metal and sulphur dioxide gas. This is one of the methods used to reduce sulphide ore of less reactive copper to copper metal.
When copper is oxidized what forms CuO?
What Does Oxidized Copper Mean? Oxidized copper is a specific type of corrosion that is produced during a three-step process where copper oxidizes to copper oxide, then to cuprous or cupric sulfide, and finally to copper carbonate.
What is copper oxidation called?
Oxidized Copper If only one atom of copper bonds to an oxygen molecule, it is called cupric oxide. If two copper atoms bond to an oxygen atom, it is cuprous oxide. Cupric oxide is considered "fully oxidized," while cuprous oxide is still in an active state.
What are the properties of cu2o?
Other names – Dicopper oxide, Red copper oxide, Cuprous oxideCu2OCopper(I) OxideDensity6 g/cm³Molecular Weight/ Molar Mass143.09 g/molBoiling Point1,800 °CMelting Point1,232 °C1 more row
Does Cu2O exist?
Copper oxides exist in two different forms: cupric oxide (CuO) and cuprous oxide (Cu2O), depending on the valence state of copper.
What type of mineral is Cu2O?
Cuprite is a copper oxide mineral (Cu2O) with cochineal-red, crimson-red, and black color and shining brown, red streak.
Why is Cu2O red?
It is red for the same reason why vermillion is red. It has a bandgap of about 2 eV, so that blue and green light are absorbed. And the absorption (the imaginary part of the complex refractive index) is related by Kramers-Kronig to the real part of the refractive index, which gives high reflectivity in the red.
Which method has been used to prepare the cu2o nanoparticles in the experiment?
A simple microplasma method was used to synthesize cuprous oxide (Cu2O) nanoparticles in NaCl–NaOH–NaNO3 electrolytic system. Microplasma was successfully used as the cathode and copper plate was used as the anode.
What is the oxidation of cu2o?
The oxidation number of Cu in Cu2O is +1.
Can cu2o be reduced by Coke?
Coke can be used to reduce Cu(2)O.
Can cu2o be reduced by carbon?
Carbon can reduce copper oxide to copper but not CaO to Ca.
How is copper oxide produced?
Copper (I) oxide may be produced by several methods. Most straightforwardly, it arises via the oxidation of copper metal: 4 Cu + O 2 → 2 Cu 2 O. Additives such as water and acids affect the rate of this process as well as the further oxidation to copper (II) oxides.
What is the formula for copper oxide?
Chemical compound. Copper (I) oxide or cuprous oxide is the inorganic compound with the formula Cu 2 O. It is one of the principal oxides of copper, the other being or copper (II) oxide or cupric oxide (CuO). This red-coloured solid is a component of some antifouling paints.
What is copper oxide used for?
Cuprous oxide is commonly used as a pigment, a fungicide, and an anti fouling agent for marine paints. Rectifier diodes based on this material have been used industrially as early as 1924, long before silicon became the standard. Copper (I) oxide is also responsible for the pink color in a positive Benedict's test .
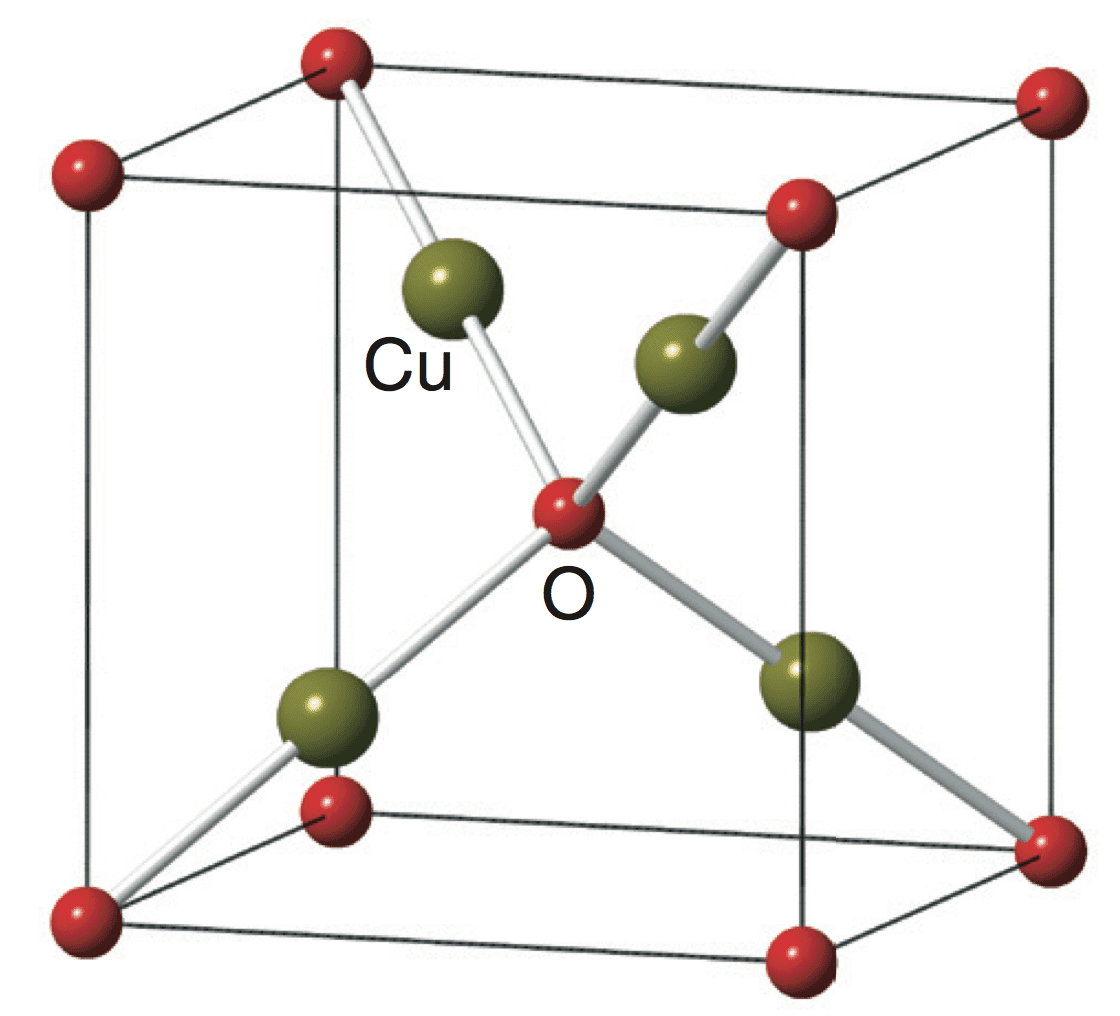
What is the space group of copper atoms?
One sublattice is shifted by a quarter of the body diagonal. The space group is Pn 3 m, which includes the point group with full octahedral symmetry.
What is the term for corrosion of silver plated copper?
It forms on silver -plated copper parts exposed to moisture when the silver layer is porous or damaged. This kind of corrosion is known as red plague .
How is Cu2O obtained?
Cu2O is obtained by oxidizing copper metal or reducing sulfur oxide copper (II) solutions, while CuO is obtained by pyrometallurgical methods used to remove copper from ores. Most of the preservatives in wood are made from copper. This is often used as a pigment to shape various glazes.
What is Copper (I) Oxide?
Copper (I) Oxide is also called as cuprous oxide an inorganic compound with the chemical formula Cu 2 O. It is covalent in nature. Copper (I) oxide crystallizes in a cubic structure. It is easily reduced by hydrogen when heated It undergoes disproportionation in acid solutions producing copper (II) ions and copper. When the cupric oxide is gently heated with metallic copper, it is converted into cuprous oxide. It acts as a good corrosion resistance, due to reactions at the surface between the copper and the oxygen in air to give a thin protective oxide layer.
What is the effect of copper on cupric oxide?
When the cupric oxide is gently heated with metallic copper, it is converted into cuprous oxide. It acts as a good corrosion resistance, due to reactions at the surface between the copper and the oxygen in air to give a thin protective oxide layer.
What is cuprous oxide used for?
Cuprous oxide is widely used in marine paints as a dye, fungicide and an antifouling agent. Industrially, rectifier diodes based on this material were used as early as 1924, long before silicon became the standard.
Is cuprous oxide soluble in water?
Cuprous oxide is practically insoluble in water and also in organic solvents. However, this compound is known to be soluble in aqueous solutions of ammonia and also in aqueous solutions of ammonium salts.
How is copper oxide formed?
It can be formed by heating copper in air at around 300–800°C: For laboratory uses, pure copper (II) oxide is better prepared by heating copper (II) nitrate, copper (II) hydroxide, or basic copper (II) carbonate:
What is copper oxide?
Copper (II) oxide dissolves in mineral acids such as hydrochloric acid, sulfuric acid or nitric acid to give the corresponding copper (II) salts: It reacts with concentrated alkali to form the corresponding cuprate salts: It can also be reduced to copper metal using hydrogen, carbon monoxide, or carbon :
What is the name of the compound that is made of copper?
Copper (II) oxide or cupric oxide is the inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu 2 O or copper (I) oxide (cuprous oxide). As a mineral, it is known as tenorite.
What is cupric oxide used for?
Cupric oxide is used as a pigment in ceramics to produce blue, red, and green, and sometimes gray, pink, or black glazes. It is incorrectly used as a dietary supplement in animal feed. Due to low bioactivity, negligible copper is absorbed. It is used when welding with copper alloys.
What is an example of natural copper oxide?
An example of natural copper (I,II) oxide is the mineral paramelaconite, Cu +2 Cu 2+2 O 3.
Which crystal system is copper oxide in?
Copper (II) oxide belongs to the monoclinic crystal system. The copper atom is coordinated by 4 oxygen atoms in an approximately square planar configuration.
What is blue color?
Used as moderate blue coloring agent in blue flame compositions with additional chlorine donors and oxidizers such as chlorates and perchlorates. Providing oxygen it can be used as flash powder oxidizer with metal fuels such as magnesium, aluminium, or magnalium powder. Sometimes it is used in strobe effects and thermite compositions as crackling stars effect.
What happens if you inhale copper fumes?
Inhalation of copper fume results in the irritation of the upper respiratory tract. ... Contact with copper fumes will also cause irritation of the eyes, nose and throat. / Copper fumes/
What is the code for copper oxide?
For Copper (II) oxide (USEPA/OPP Pesticide Code: 042401) ACTIVE products with label matches. /SRP: Registered for use in the U.S. but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses./
What are the risks of copper fumes?
Persons at special risk include those with impaired pulmonary function , especially those with obstructive airway diseases, since the breathing of copper fume might cause exacerbation of symptoms due to its irritant properties. / Copper fume/
What is the reaction of verdigris?
Verdigris, formed by atmospheric corrosion of the surface of metallic copper presumably composed of copper carbonates & oxides, causes immediate irritation & conjunctival inflammation when accidentally dropped or dusted on the eyes of patients, but the reaction subsides without permanent damage soon after the eye is cleaned by irrigation. /Verdigris/
How is copper oxide formed?
The most common way copper (I) oxide is formed by oxidation of the copper metal.
What is the shape of cuprous oxide?
Crystals of cuprous oxide are found in cubic shape. When you heat the solution of Cu2O in the presence of hydrogen, the solution is reduced quickly. It is disproportionated in the solution of acid and produces copper and copper (II) ions. Cupric oxide, when heated with the metallic copper, is turned into cuprous oxide.
What is the difference between cupric acid and cuprous oxide?
Cupric oxide is a brown colored powder while cuprous oxide is a red coloured. When an atom of the copper band is attached to an oxygen molecule, then it is cupric acid. When an oxygen atom is attached to two copper atoms, it is said to be cuprous oxide.
What is the chemical reaction between hydrogen chloride and copper oxide?
Through the chemical reaction between hydrogen chloride and copper (I) oxide, Copper (I) Chloride is formed. Well, Oxygen of Copper (I) Oxide is reduced with chlorine atoms and form the copper chloride relatively. You can understand the chemical reaction between hydrogen chloride and Cu2O from the below chemical equation.
What is the color of copper oxide?
Colour of copper oxide is a bit confusing as sometimes you might have seen red or black coloured copper oxides. Well, here you should have a clear idea that there are two types of copper oxides like copper (I) oxide that is black in colour and copper (II) oxide that is red.
How does copper oxide react with water?
Copper ( I) Oxide can react with water as the oxygen is present in the water and make Copper (II) Hydroxide. Following is the chemical equation to understand the chemical reaction of copper (I) oxide and water. 2Cu2O + 4H2O + O2 → 4Cu (OH)2. Through the chemical reaction between hydrogen chloride and copper (I) oxide, Copper (I) Chloride is formed.
What is the chemical structure of copper oxide?
Copper Oxide where copper is in liquid form is called cuprous oxide. Cu2O is the chemical structure of cuprous oxide. Well, here in Cu2O copper and oxygen share a covalent bond; hence it naturally has covalent bonds. Crystals of cuprous oxide are found in cubic shape. When you heat the solution of Cu2O in the presence of hydrogen, the solution is reduced quickly. It is disproportionated in the solution of acid and produces copper and copper (II) ions. Cupric oxide, when heated with the metallic copper, is turned into cuprous oxide. In the presence of moisture in the air, oxygen reacts with copper on the surface of any object and cuprous oxide can act as corrosion resistance in such condition. It will serve as the protective layer of oxide that is thin.
What are the physical properties of CuO?
CuO has attracted particular attention because it is the simplest member of the family of copper compounds and exhibits a range of potentially useful physical properties, such as high temperature superconductivity, electron correlation effects, and spin dynamics [61,62].
How is flotation of copper minerals done?
The process involves two basic flotation methods: (a) fatty acid flotation of oxide copper minerals from siliceous ore, and (b) sulphidization of oxide copper minerals followed by flotation using sulfhydryl collectors , such as xanthate [1] from carbonate ores. In the past 50 years, extensive research has been carried out on a variety of oxide copper minerals, and only a few of the many innovative processes have been introduced into operating plants. It was not until recently that new technology has been developed and introduced into some operating plants around the world. One of the major problems with flotation of oxide copper minerals, at industrial scale, is that the floatability of oxide copper minerals from natural ores depends largely on the mineralogy of the ore and the gangue composition. The floatability of oxide copper minerals that are present in the ore containing carbonaceous and dolomitic gangue is significantly different from the flotation properties of oxide copper containing siliceous gangue minerals.
What is the method used to treat gold containing oxide copper ores?
Recently, a new class of collectors, based on ester-modified xanthates, have been successfully used to treat gold-containing oxide copper ores, using a sulphidization method . Table 17.6 compares the metallurgical results obtained on the Igarape Bahia ore using xanthate and a new collector (PM230, supplied by Senmin in South Africa).
What are the effects of copper oxide nanoparticles on bacteria?
Copper oxide nanoparticles (CuONPs) have toxic effects against many microbes by generating the reactive oxygen species that disrupts DNA and amino acid synthesis. It has been revealed that CuONPs have high affinity to carboxyl and amine groups on the surface of B. subtilis cells, this affinity being higher than the interaction of silver NPs with these bacteria cells. CuONPs are also efficient against a range of resistant bacteria pathogens, including MRSA and multidrug-resistant E. coli ( Esteban et al., 2009 ).
What is the octahedral site of copper oxide?
Copper oxides are unusual in two respects. First, octahedral-site Cu (II):t6e3 contains a single e hole in the 3 d shell , which makes it orbitally degenerate and therefore a strong Jahn–Teller ion; consequently, Cu (II) ions normally occupy octahedral sites that are deformed to tetragonal ( c /a > 1) symmetry by Jahn–Teller orbital ordering. However, in the absence of a cooperativity that stabilizes long-range orbital ordering, the electrons may couple locally to E-mode vibrations, forming vibronic states in a dynamic Jahn–Teller coupling. Second the Cu (II):3 d9 energy level lies below the top of the O 2 − :2 p6 valence band in an ionic model; the introduction of covalent bonding creates states of e -orbital symmetry at the top of the O 2 − :2 p6 bands that have a large o 2 pσ component (see Fig. 26 ). Locally this o 2 pσ component increases dramatically on oxidation of Cu (II) to Cu (III). The change in hybridization represents a polarization of the oxygen atoms that decreases the equilibrium Cu O bond length, but the change in polarization is fast relative to the motion of the oxygen nucleus. Therefore, a dynamic vibronic phenomenon may reflect coupling to the polarization cloud of the oxygen atoms rather than to significant oxygen-atom displacements. Nevertheless, hybridization with a polarization wave on the oxygen-atom array would significantly increase the effective mass m* of an itinerant electron.
What is the symmetry of copper oxides?
First, octahedral-site CuII :t 6e3 contains a single e hole in the 3 d shell, which makes it orbitally degenerate and therefore a strong Jahn–Teller ion; consequently, Cu II ions normally occupy square coplanar, pyramidal, or octahedral sites that are deformed to tetragonal ( c /a > 1) symmetry by Jahn–Teller orbital ordering. However, in the absence of a cooperativity that stabilizes long-range orbital ordering, the electrons may couple locally to E-mode vibrations, forming vibronic states in a dynamic Jahn–Teller coupling. Second, the Cu II :3 d9 energy level lies below the top of the O 2 − :2 p6 valence band in an ionic model; the introduction of covalent bonding creates states of e -orbital symmetry at the top of the O 2 − :2 p6 bands that have a large O-2 pσ component (see Figure 26 ). Locally this O-2 pσ component increases dramatically on oxidation of Cu II to Cu III. The change in orbital mixing represents a polarization of the oxygen atoms that decreases the equilibrium Cu-O bond length, but the change in polarization is fast relative to the motion of the oxygen nucleus. Therefore, a dynamic vibronic phenomenon may reflect coupling to the polarization cloud of the oxygen atoms rather than to significant oxygen-atom displacements. Nevertheless, hybridization of an atomic vibration wave with a polarization wave on the oxygen-atom array would significantly increase the effective mass m * of an itinerant electron.
What are the problems with flotation of copper?
One of the major problems with flotation of oxide copper minerals, at industrial scale, is that the floatability of oxide copper minerals from natural ores depends largely on the mineralogy of the ore and the gangue composition. The floatability of oxide copper minerals that are present in the ore containing carbonaceous ...
How is CuO obtained?
CuO is generally obtained via the oxidation of copper and it can have yellow or red color. Cu2O degrades to CuO in moist air.
What is the formula for copper oxide?
Cuprous oxide has the formula Cu2O. In cuprous oxide, the oxidation state of copper is +1. It is a reddish brown powder. Used as pigment, fungicide, etc.
What reacts with calcium oxide in cement?
Other naturally-occurring acidic/amphoteric oxides such as Fe2O3 and Al2O3 also react with calcium oxide in the cement kiln to form the minerals that cause cement to set (3CaO.Al2O3) and act as a flux in the kiln to lower the temperature of reaction (4CaO.Al2O3.Fe2O3). (Both of these latter minerals are liquid above about 1200C, and this liquid helps to facilitate the silicate reactions shown above.
What is copper called?
Copper, which is a d block element, is named as cuprous or cupric based on the electronic configuration.
What is cupric oxide used for?
Cupric oxide is used as a pigment in ceramics to produce blue, red, and green, and sometimes gray, pink, or black glazes. It is also incorrectly used as a dietary supplement in animal feed. Due to low bioactivity, negligible copper is absorbed. It is also used when welding with copperalloys.
What is a simple oxide?
Simple oxides are Oxides that carry only that number of oxygen atoms as is allowed by the normal valency of the element or metal.
When copper is reacted with oxygen, what are the two stable compounds?
When copper is reacted with oxygen, two stable compounds Cu2O and CuO form.
Abstract
Cuprous oxide (Cu 2 O) nanoparticles have been successfully synthesized using copper acetate as precursor via supersaturation theory as a facile rout. Synthesis parameters, such as the reducing agent concentration, reaction temperature, reaction time, type of the reducing agent and rate of adding reducing agent were investigated.
Keywords
In het rijk van de ideeën hangt alles af van het enthousiasme... in de echte wereld alle rust op doorzettingsvermogen… “In the realm of ideas everything depends on enthusiasm... in the real world all rests on perseverance…” J.W. von Goethe (1749–1832)
Introduction
Metal oxides play an inconceivable role and are of paramount importance in many rapidly developing research areas such as the intersection of chemistry and materials science [1]. Potential applications include sensors, microelectronics, corrosion protection coatings, fuel cells, nanotechnology in general or catalysis [1], [2].
Experimental
Copper acetate (Cu (CH 3 COO) 2 ·H 2O), sodium hydroxide (NaOH), ascorbic acid (C6 H 8 O 6) and maltodextrin (C 6 H 12 O 6) were purchased from Sigma–Aldrich Co., Germany. All the chemicals were in analytical grade and used without further purification. Deionized water was used in the experiments.
Results and discussion
XRD pattern of the reducing agent adding effect is illustrated in Fig. 2. As can be seen from this figure, comparing the results of the peaks show synthesized samples are matched to principal patterns of peaks in the X’Pert software, by reference model of (00-005-0667).
Conclusion
Cuprous oxide (Cu 2 O) nanoparticles have been successfully synthesized using copper acetate as precursors and acetic acid as reducing agent via supersaturation method. Effects of the influential parameters like temperature, type of the reducing agent, concentration of reducing agent were investigated.
Acknowledgment
Authors gratefully thank the Zanjan Zinc Khalessazan Industries Company (ZZKICO), for any supports.
Overview
Preparation
Copper(I) oxide may be produced by several methods. Most straightforwardly, it arises via the oxidation of copper metal:
4 Cu + O2 → 2 Cu2O
Additives such as water and acids affect the rate of this process as well as the further oxidation to copper(II) oxides. It is also produced commercially by reduction of copper(II) solutions with su…
Reactions
Aqueous cuprous chloride solutions react with base to give the same material. In all cases, the color is highly sensitive to the procedural details.
Formation of copper(I) oxide is the basis of the Fehling's test and Benedict's test for reducing sugars. These sugars reduce an alkaline solution of a copper(II) salt, giving a bright red precipitate of Cu2O.
Properties
The solid is diamagnetic. In terms of their coordination spheres, copper centres are 2-coordinated and the oxides are tetrahedral. The structure thus resembles in some sense the main polymorphs of SiO2, and both structures feature interpenetrated lattices.
Copper(I) oxide dissolves in concentrated ammonia solution to form the colourless complex [Cu(NH3)2] , which is easily oxidized in air to the blue [Cu(NH3)4(H2O)2] . It dissolves in hydrochlo…
Structure
Cu2O crystallizes in a cubic structure with a lattice constant al = 4.2696 Å. The copper atoms arrange in a fcc sublattice, the oxygen atoms in a bcc sublattice. One sublattice is shifted by a quarter of the body diagonal. The space group is Pn3m, which includes the point group with full octahedral symmetry.
Semiconducting properties
In the history of semiconductor physics, Cu2O is one of the most studied materials, and many experimental semiconductor applications have been demonstrated first in this material:
• Semiconductor
• Semiconductor diodes
• Phonoritons ("a coherent superposition of exciton, photon, and phonon")
Applications
Cuprous oxide is commonly used as a pigment, a fungicide, and an antifouling agent for marine paints. Rectifier diodes based on this material have been used industrially as early as 1924, long before silicon became the standard. Copper(I) oxide is also responsible for the pink color in a positive Benedict's test.
In December 2021, Toshiba announced the creation of a transparent cuprous oxide (Cu2O) thin-fi…
Similar compounds
An example of natural copper(I,II) oxide is the mineral paramelaconite, Cu4O3 or Cu 2Cu 2O3.
Overview
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds.
Production
It is produced on a large scale by pyrometallurgy, as one stage in extracting copper from its ores. The ores are treated with an aqueous mixture of ammonium carbonate, ammonia, and oxygen to give copper(I) and copper(II) ammine complexes, which are extracted from the solids. These complexes are decomposed with steam to give CuO.
It can be formed by heating copper in air at around 300–800°C:
Reactions
Copper(II) oxide dissolves in mineral acids such as hydrochloric acid, sulfuric acid or nitric acid to give the corresponding copper(II) salts:
CuO + 2 HNO3 → Cu(NO3)2 + H2O CuO + 2 HCl → CuCl2 + H2O CuO + H2SO4 → CuSO4 + H2O
It reacts with concentrated alkali to form the corresponding cuprate salts:
2 MOH + CuO + H2O → M2[Cu(OH)4]
Structure and physical properties
Copper(II) oxide belongs to the monoclinic crystal system. The copper atom is coordinated by 4 oxygen atoms in an approximately square planar configuration.
The work function of bulk CuO is 5.3 eV
Uses
As a significant product of copper mining, copper(II) oxide is the starting point for the production of other copper salts. For example, many wood preservatives are produced from copper oxide.
Cupric oxide is used as a pigment in ceramics to produce blue, red, and green, and sometimes gray, pink, or black glazes.
It is incorrectly used as a dietary supplement in animal feed. Due to low bioactivity, negligible co…
Similar compounds
An example of natural copper(I,II) oxide is the mineral paramelaconite, Cu 2Cu 2O3.
See also
• Patina
External links
• National Pollutant Inventory - Copper and compounds fact sheet
• Copper oxides project page
• CDC - NIOSH Pocket Guide to Chemical Hazards