How is norepinephrine removed from active sites?
Norepinephrine is removed from active sites by a variety of mechanisms. Generally, norepinephrine is removed by active reuptake into axon terminals, where it either is metabolized or re-enters storage granules.
What happens to norepinephrine after it leaves the synapse?
Once in the synapse, norepinephrine binds to and activates receptors. After an action potential, the norepinephrine molecules quickly become unbound from their receptors. They are then absorbed back into the presynaptic cell, via reuptake mediated primarily by the norepinephrine transporter (NET).
How are neurotransmitters removed from the synapse?
So these pumps are called "Re-uptake Pumps," because they take the neurotransmitter back into the axon terminal where it came from in the first place. By doing so, they remove the neurotransmitter from the synaptic cleft. Another big method of actively removing neurotransmitter from the synapse, is by "Astrocyte Endfeet."
How is norepinephrine converted to epinephrine in the brain?
In the adrenal medulla and in a few brain regions, norepinephrine is converted to epinephrine by the enzyme phenylethanolamine N -methyltransferase. The biosynthesis of the catecholamines is summarized in Fig. 2. Figure 2.
See more

How is norepinephrine removed from synaptic?
Norepinephrine can then be released from the presynaptic terminal to the synaptic cleft via exocytosis or convert to epinephrine in neurons that contain the enzyme phenylethanolamine-N-methyl transferase.
How is norepinephrine terminated?
Norepinephrine activity is efficiently terminated through inactivation by the enzymes catechol-O-methyltransferase (COMT) or monoamine oxidase (MAO), by reuptake into nerve endings, or by diffusion from binding sites.
How are neurotransmitters removed from synapses?
Some neurotransmitters are removed from the synaptic cleft by special transporter proteins on the pre-synaptic membrane.
How is norepinephrine produced and eliminated?
[7] It is released from the adrenal medulla into the blood as a hormone, and is also a neurotransmitter in the central nervous system and sympathetic nervous system where it is released from noradrenergic neurons. The actions of norepinephrine are carried out via the binding to adrenergic receptors.
Where is norepinephrine metabolized?
Norepinephrine is contained in complex systems of specific neurons and is highly localized at nerve terminals, largely within synaptic vesicles. The metabolism of norepinephrine in the brain is generally similar to that in the peripheral sympathetic system.
Where are epinephrine and norepinephrine degraded?
Catecholamines Are Degraded Rapidly Both epinephrine and norepinephrine remain in the circulation only a very short time: their half-lives are about 1–3 min and their metabolic clearances are from 2 to 6 L min−1. Circulating epinephrine is degraded mostly in the liver and the kidney.
How are serotonin and other catecholamines removed from the synapses?
Indolamines (serotonin and tryptamine) are synthesized from tryptophan (Figure 2.3.). Action of catecholamines and indolamines on target cells is terminated much more slowly than those of acetylcholine; they are removed from synaptic cleft by reuptake.
How are neurotransmitters removed from the synaptic cleft quizlet?
What are the two mechanisms by which neurotransmitters can be removed from the synaptic cleft? (1) degradation - neurotransmitter is chemically inactivated in synaptic cleft (ex. ACh), (2) reuptake - neurotransmitter is reabsorbed by a neurotransmitter transport protein in the membrane of the presynaptic neuron. 3.
What molecule removes neurotransmitters from the synaptic cleft?
3. Glial cells: astrocytes remove neurotransmitters from the synaptic cleft. 4. Reuptake: the whole neurotransmitter molecule is taken back into the axon terminal that released it.
How is epinephrine eliminated from the body?
Epinephrine is rapidly inactivated mainly by enzymic transformation to metanephrine or normetanephrine, either of which is then conjugated and excreted in the urine in the form of both sulfates and glucuronides.
How are catecholamines eliminated?
Dopamine, epinephrine (adrenaline), and norepinephrine are the main catecholamines. Each of these hormones gets broken down into other substances that are eliminated in your urine.
Which neurons release norepinephrine?
postganglionic neuronsNorepinephrine gets released by postganglionic neurons of the sympathetic nervous system, which binds to and activates adrenergic receptors. These receptors further divide into alpha11 (G coupled receptor), alpha-2 (G coupled receptor), beta-1 (G coupled receptor), and beta-2 and beta-3 (G and G coupled receptor).
What is the role of dopamine and norepinephrine in the synaptic cleft
The reuptake of norepinephrine and dopamine is essential in regulating the concentration of monoamine neurotransmitters in the synaptic cleft. The transporter also helps maintain homeostatic balances of the presynaptic neuron. Norepinephrine structure.
Where are norepinephrine transporters located?
They are found closer to the plasma membrane of the cell. This requires norepinephrine to diffuse from the site it is released to the transporter for reuptake. Norepinephrine transporters are confined to the neurons of the sympathetic system, and those innervating the adrenal medulla, lung, and placenta.
What is the role of NE in the nervous system?
NE, also known as noradrenaline (NA), has an important role in controlling mood, arousal, memory, learning, and pain perception. NE is a part of the sympathetic nervous system.
What is the epigenetic mechanism of CPG islands?
An epigenetic mechanism (hypermethylation of CpG islands in the NET gene promoter region) that results in reduced expression of the noradrenaline (norepinephrine) transporter and consequently a phenotype of impaired neuronal reuptake of norepinephrine has been implicated in both postural orthostatic tachycardia syndrome and panic disorder.
What is a single nucleotide polymorphism?
A single-nucleotide polymorphism (SNP) is a genetic variation in which a genome sequence is altered by a single nucleotide ( A, T, C or G ). NET proteins with an altered amino acid sequence (more specifically, a missense mutation) could potentially be associated with various diseases that involve abnormally high or low plasma levels of norepinephrine due to altered NET function. NET SNPs and possible associations with various diseases are an area of focus for many research projects. There is evidence suggesting a relationship between NET SNPs and various disorders such as ADHD psychiatric disorders, postural tachycardia and orthostatic intolerance. The SNPs rs3785143 and rs11568324 have been related to attention-deficit hyperactivity disorder. Thus far, however, the only confirmed direct association between a SNP and a clinical condition is that of the SNP, Ala457Pro, and orthostatic intolerance. Thirteen NET missense mutations have been discovered so far.
What are the effects of post-translational modifications on the function of the net?
Post-translational modifications can have a wide range of effects on the function of the NET, including the rate of fusion of NET-containing vesicles with the plasma membrane, and transporter turnover.
What is the effect of depletion of noradrenaline on the brain?
Depletion of noradrenaline (norepinephrine) in the brain has been shown to cause a decrease in drive and motivation and might be linked to depression. It is part of the ‘fight or flight’ response, which increases heart rate, etc. View chapter Purchase book. Read full chapter.
How is noradrenaline inacitvated?
Noradrenaline is inacitvated by its removal from the synaptic cleft. Two distinct uptake systems for noradrenaline have been identified. The cocaine-sensitive transport of noradrenaline (uptake 1) occurs in adrenergic neurones ( Graefe and Bönisch 1988) and the corticosterone-sensitive transport (uptake 2) in extraneuronal tissues such as myocardium, salivary glands, or vascular smooth muscle ( Trendelenburg 1988 ). In the past, uptake 2 has mainly been investgated in isolated organs. A model for uptake 2 which is based on a clonal cell line has not been described so far.
What is NA in neurotransmitters?
NA is a major neurotransmitter of the ANS, especially in sympathetic neurons, and fundamentally mediates the function of the ANS. Consistent with this, a rare human disease called the dopamine β-hydroxylase deficient disease, in which NA is undetectable, was identified to be associated with severe autonomic function failure [28]. NA is one of the catecholamine neurotransmitters that are synthesized from tyrosine by three consecutive enzymatic steps. While tyrosine hydroxylase is responsible for the first step of catecholamine biosynthesis, converting tyrosine to L-dopa, and is expressed in all catecholamine neurons, dopamine β-hydroxylase (DBH) is responsible for conversion of dopamine to NA and is specifically expressed in NA neurons. Thus, DBH is a hallmark protein of NA neurons and the control mechanism of its expression is an essential feature of the development of NA neurons.
What is the methylated form of noradrenaline?
Through various intermediate steps ( Figure 1 ), l -tyrosine is converted to noradrenaline and, finally, to its methylated form, adrenaline, in phenylethanolamine N -methyltransferase-containing cells. The first cytosolic enzyme, tyrosine hydroxylase, is inhibited by excess production of noradrenaline and is the rate-limiting step in the regulation of noradrenaline synthesis. Tyrosine hydroxylase is found only in the cytosol in catecholamine-containing cells and is used as a marker for detection of adrenergic neurons. Dopamine β -hydroxylase is also used as a selective marker for catecholamine-containing cells, but it is located in the secretory vesicles, usually membrane bound; however, a small amount is soluble and released with the vesicle contents upon exocytosis.
What is the name of the neurotransmitter in the peripheral nervous system?
Norepinephrine. Norepinephrine (also called noradrenaline) is a neurotransmitter in both the peripheral nervous system and central nervous system and a hormone. From: Reference Module in Biomedical Sciences, 2016. Download as PDF. About this page.
What is the NE level in a rat?
NE, an important neurotransmitter, plays a vital role in a variety of functions, and it begins to develop rather early in life. NE content in the newborn rat appears to vary between 18% and 29% of the adult value in the whole brain. 19,20,25 However, by 2 weeks of age, concentrations of NE in the whole brain reach 55%–85% of the adult level.
How long does it take for NE to reach adult levels?
24 Finally, it takes 35–45 days to reach the adult level of NE in the frontal cortex and hippocampus. View chapter Purchase book. Read full chapter.
Where is epinephrine synthesized?
Epinephrine is synthesized from norepinephrine within the adrenal medulla, which are small glands associated with the kidneys. Preganglionic fibers of the sympathetic nervous system synapse within the adrenals. Activation of these preganglionic fibers releases acetylcholine, which binds to postjunctional nicotinic receptors in the tissue. This leads to stimulation of NE synthesis within adenomedullary cells, but unlike sympathetic neurons, there is an additional enzyme (phenylethanolamine-N-methyltransferase) that adds a methyl group to the NE molecule to form epinephrine. The epinephrine is released into the blood perfusing the glands and carried throughout the body.
What is the primary neurotransmitter for postganglionic sympathetic adrenergic nerves?
Norepinephrine (NE) is the primary neurotransmitter for postganglionic sympathetic adrenergic nerve s. It is synthesized inside the nerve axon, stored within vesicles, then released by the nerve when an action potential travels down the nerve. Below are the details for release and synthesis of NE:
Does NE leave the receptor?
If the nerve stops releasing NE, then the NE concentration in the junctional cleft will decrease and NE will leave the receptor. There are several mechanisms by which the norepinephrine is removed from the intercellular (junctional) space and therefore from the postjunctional receptor:
Which pathway does norepinephrine follow?
Norepinephrine follows the same pathway as dopamine. Reuptake into the presynaptic terminal occurs via the norepinephrine transporter (NET), and then the transmitter is either degraded within the cell by MAO or COMT or repackaged into synaptic vesicles.
What happens after neurotransmitters are released into the synaptic cleft?
After neurotransmitters have been released into the synaptic cleft, they act upon postsynaptic receptors, as covered in the previous chapters. That action must be terminated in order for proper neuronal communication to continue. This is accomplished mainly through two processes: neurotransmitter transport and/or degradation. Transport physically removes the neurotransmitter molecule from the synaptic cleft. Degradation breaks down the neurotransmitter molecule by enzyme activity.
How does dopamine work?
Dopamine. Dopamine action is terminated by reuptake into the presynaptic terminal via the dopamine transporter (DAT). Once inside the cell, dopamine is either degraded via the actions of either monoamine oxidase (MAO) or catechol-O-methyltransferase (COMT), or it is repackaged into vesicles. Figure 13.4.
What enzyme degrades acetylcholine?
Acetylcholine action is terminated by acetylcholinesterase, an enzyme present in the synaptic cleft. Acetylcholinesterase degrades acetylcholine into choline and acetate molecules. Choline is then transported back into the presynaptic terminal and used in the synthesis of new acetylcholine.
Where is serotonin transported?
Like the other monoamines, serotonin is transported back into the presynaptic terminal via the serotonin transporter (SERT). The difference between serotonin and the catecholamines dopamine and norepinephrine is that monoamine oxidase is the only enzyme used for degradation.
Where does glutamate reuptake?
Reuptake of glutamate molecules into the presynaptic terminal can occur, or glutamate can be transported into nearby glial cells. The excitatory amino acid transporters are sodium co-transporters and use the sodium electrochemical gradient to drive neurotransmitter transport.
How does neurotransmitter transport work?
This is accomplished mainly through two processes: neurotransmitter transport and/or degradation. Transport physically removes the neurotransmitter molecule from the synaptic cleft. Degrada tion breaks down the neurotransmitter molecule by enzyme activity.

Overview
Functions
Like many other biologically active substances, norepinephrine exerts its effects by binding to and activating receptors located on the surface of cells. Two broad families of norepinephrine receptors have been identified, known as alpha and beta adrenergic receptors. Alpha receptors are divided into subtypes α1 and α2; beta receptors into subtypes β1, β2, and β3. All of these function as G protein-co…
Structure
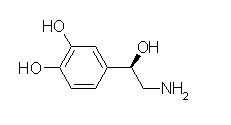
Norepinephrine is a catecholamine and a phenethylamine. Its structure differs from that of epinephrine only in that epinephrine has a methyl group attached to its nitrogen, whereas the methyl group is replaced by a hydrogen atom in norepinephrine. The prefix nor- is derived as an abbreviation of the word "normal", used to indicate a demethylated compound. Norepinephrine comprises of a cat…
Biochemical mechanisms
Norepinephrine is synthesized from the amino acid tyrosine by a series of enzymatic steps in the adrenal medulla and postganglionic neurons of the sympathetic nervous system. While the conversion of tyrosine to dopamine occurs predominantly in the cytoplasm, the conversion of dopamine to norepinephrine by dopamine β-monooxygenase occurs predominantly inside ne…
Pharmacology
A large number of important drugs exert their effects by interacting with norepinephrine systems in the brain or body. Their uses include treatment of cardiovascular problems, shock, and a variety of psychiatric conditions. These drugs are divided into: sympathomimetic drugs which mimic or enhance at least some of the effects of norepinephrine released by the sympathetic nervous system; sympatholytic drugs, in contrast, block at least some of the effects. Both of these are lar…
Diseases and disorders
A number of important medical problems involve dysfunction of the norepinephrine system in the brain or body.
Hyperactivation of the sympathetic nervous system is not a recognized condition in itself, but it is a component of a number of conditions, as well as a possible consequence of taking sympathomimetic drugs. It causes a distinctive set of symptoms including aches and pains, rapi…
Comparative biology and evolution
Norepinephrine has been reported to exist in a wide variety of animal species, including protozoa, placozoa and cnidaria (jellyfish and related species), but not in ctenophores (comb jellies), whose nervous systems differ greatly from those of other animals. It is generally present in deuterostomes (vertebrates, etc.), but in protostomes (arthropods, molluscs, flatworms, nematodes, annelids, etc.) it is …
History
Early in the twentieth century Walter Cannon, who had popularized the idea of a sympathoadrenal system preparing the body for fight and flight, and his colleague Arturo Rosenblueth developed a theory of two sympathins, sympathin E (excitatory) and sympathin I (inhibitory), responsible for these actions. The Belgian pharmacologist Zénon Bacq as well as Canadian and US-American pharmacologists between 1934 and 1938 suggested that noradrenaline might be a sympathetic …
Overview
The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene.
NET is a monoamine transporter and is responsible for the sodium-chloride (Na /Cl )-dependent reuptake of extracellular norepinephrine (NE), which is also kno…
Gene
The norepinephrine transporter gene, SLC6A2 is located on human chromosome 16 locus 16q12.2. This gene is encoded by 14 exons. Based on the nucleotide and amino acid sequence, the NET transporter consists of 617 amino acids with 12 membrane-spanning domains. The structural organization of NET is highly homologous to other members of a sodium/chloride-dependent family of neurotransmitter transporters, including dopamine, epinephrine, serotonin and GABA tra…
Structure
The norepinephrine transporter is composed of 12 transmembrane domains (TMDs). The intracellular portion contains an amino (-NH 2) group and carboxyl (-COOH) group. In addition, there is a large extracellular loop located between TMD 3 and 4. The protein is composed of 617 amino acids.
Function
NET functions to transport synaptically released norepinephrine back into the presynaptic neuron. As much as 90% of the norepinephrine released will be taken back up in the cell by NET. NET functions by coupling the influx of sodium and chloride (Na /Cl ) with the transport of norepinephrine. This occurs at a fixed ratio of 1:1:1. Both the NET and the dopamine transporter (DAT) can transport …
Transport mechanisms
The transport of norepinephrine back into presynaptic cell is made possible by the cotransport with Na and Cl . The sequential binding of the ions results in the eventual reuptake of norepinephrine. The ion gradients of Na and Cl make this reuptake energetically favorable. The gradient is generated by the Na+/K+-ATPase which transports three sodium ions out and two potassium ions into the cell. NETs have conductances similar to those of ligand-gated ion chann…
Location in the nervous system
NETs are restricted to noradrenergic neurons and are not present on neurons that release dopamine or epinephrine. The transporters can be found along the cell body, axons, and dendrites of the neuron. NETs are located away from the synapse, where norepinephrine is released. They are found closer to the plasma membrane of the cell. This requires norepinephrine to diffuse from the site it is released to the transporter for reuptake. Norepinephrine transporters are confined t…
Regulation
Regulation of NET function is complex and a focus of current research. NETs are regulated at both the cellular and molecular level post-translation. The most understood mechanisms include phosphorylation by the second messenger protein kinase C (PKC). PKC has been shown to inhibit NET function by sequestration of the transporter from the plasma membrane. The amino acid sequence of NET has shown multiple sites related to protein kinase phosphorylation. Post-transl…
Clinical significance
Orthostatic intolerance (OI) is a disorder of the autonomic nervous system (a subcategory of dysautonomia) characterized by the onset of symptoms upon standing. Symptoms include fatigue, lightheadedness, headache, weakness, increased heart rate/heart palpitations, anxiety, and altered vision. Often, patients have high plasma norepinephrine (NE) concentrations (at least 600 pg/ml) in relation to sympathetic outflow upon standing, suggesting OI is a hyperadrenergic condition. …