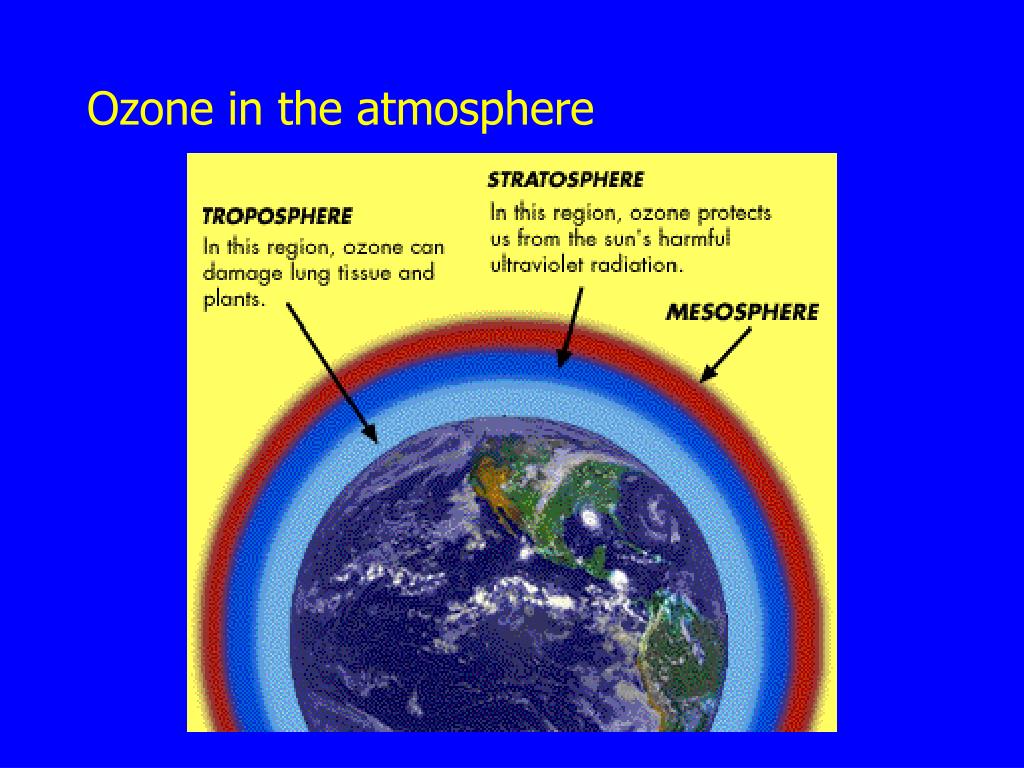
What role does ozone play in the atmosphere?
The ozone layer shields the earth from the sun's harmful UV rays. Over the past ten years, scientists worldwide have recorded decreasing levels of ozone in the atmosphere. Less ozone means that more UV radiation reaches earth, increasing the danger of sun damage.
How does ozone build up in the atmosphere?
Ozone production from NOx pollutants: Oxygen atoms freed from nitrogen dioxide by the action of sunlight attack oxygen molecules to make ozone. Nitrogen oxide can combine with ozone to reform nitrogen dioxide, and the cycle repeats. Ozone can also be found in the troposphere, the lowest layer of the atmosphere.
What does the ozone do in your atmosphere?
Ozone is only a trace gas in the atmosphere—only about 3 molecule s for every 10 million molecules of air. But it does a very important job. Like a sponge, the ozone layer absorbs bits of radiation hitting Earth from the sun. Even though we need some of the sun's radiation to live, too much of it can damage living things.
What are facts about ozone?
- 1. Ozone was first discovered and isolated by the German-Swiss chemist Christian Friedrich Schönbein in 1839.
- 2. Ozone is a pale blue gas with a distinctive pungent smell.
- 4. One molecule of ozone consists of three oxygen atoms. ...
- 5. ...
- 6. ...
- 7. ...
- 8. ...
- 9. ...
- 10. ...
See more

How is ozone formed in the upper layer of atmosphere?
Ozone is formed in upper atmosphere by the reaction of ultraviolet UV radiations on oxygen O2 molecule. The damage to ozone layer is a cause of concern to us as due to its damage more ultraviolet rays reach the earth's surface causing various health hazards.
How is ozone formed in the upper atmosphere Class 9?
Complete answer: Ozone is formed in the stratosphere naturally by solar ultraviolet radiations (UV) and oxygen molecules. It makes up about 21% of our atmosphere. These reactions take place continuously whenever solar radiation is falling on them due to which the largest amount of ozone is formed in this layer itself.
How is ozone formed in the atmosphere Class 10?
Atmospheric oxygen is decomposed by absorbing ultraviolet rays having high energy. These oxygen atoms combine with oxygen molecules to form ozone molecules.
Why is ozone found in upper atmosphere and not on Earth?
Most air molecules are either oxygen (O2) or nitrogen (N2) molecules. In the troposphere near Earth's surface, ozone is even less abundant, with a typical range of 20 to 100 ozone molecules for each billion air mole- cules. The highest surface values result when ozone is formed in air polluted by human activities.
Why ozone is important in the upper atmosphere?
The ozone layer acts as a shield for life on Earth. Ozone is good at trapping a type of radiation called ultraviolet radiation, or UV light, which can penetrate organisms' protective layers, like skin, damaging DNA molecules in plants and animals.
Where is ozone naturally produced in the atmosphere?
the stratosphereOzone is naturally produced in the stratosphere by a two- step reactive process. In the first step, solar ultraviolet radiation (sunlight) breaks apart an oxygen molecule to form two separate oxygen atoms.
How is ozone produced in the upper atmosphere quizlet?
Ozone is produced naturally in the stratosphere when highly energetic solar radiation strikes molecules of oxygen, O2, and cause the two oxygen atoms to split apart in a process called photolysis. If a freed atom collides with another O2, it joins up, forming ozone O3.
Why ozone is not formed below stratosphere?
Because the ozone absorbs the higher-energy UV photons, no significant amount of ozone forms in the lower part of the atmosphere.
Why is ozone only in the stratosphere?
The natural level of ozone in the stratosphere is a result of a balance between sunlight that creates ozone and chemical reactions that destroy it. Ozone is created when the kind of oxygen we breathe—O2—is split apart by sunlight into single oxygen atoms.
What is ozone how is it formed in the atmosphere explain with equation?
Formation of Ozone : The UV radiations split molecular oxygen O2 apart into free oxygen atoms O + O. These atoms then combine with molecular oxygen to form ozone. O2 → O + O O + O2 → O3Effect : Ozone layer shields the surface of the earth from damaging UV radiations of the sun. Related Answer.
What are the causes of ozone depletion Class 9?
Chlorofluorocarbons or CFCs are the main cause of ozone layer depletion. These are released by solvents, spray aerosols, refrigerators, air-conditioners, etc. The molecules of chlorofluorocarbons in the stratosphere are broken down by ultraviolet radiations and release chlorine atoms.
What Is Ozone and Where Is It in The atmosphere?
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made product that occurs in the Earth's upper at...
Are High Ambient Ozone Concentrations Found only in Heavily Urbanized areas?
Many people mistakenly believe that tropospheric ozone concentrations are high only in major urban areas, but high ambient ozone concentrations can...
How Does Atmospheric Ozone Affect Human Health?
Ozone has two properties of interest to human health. First, it absorbs UV light, reducing human exposure to harmful UV radiation that causes skin...
What is ozone and where is it in the atmosphere?
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made product that occurs in the Earth's upper atmosphere
Where does ozone come from?
Although some stratospheric ozone is transported into the troposphere, and some VOC and NOx occur naturally, the majority of ground-level ozone is the result of reactions of man-made VOC and NOx. Significant sources of VOC are chemical plants, gasoline pumps, oil-based paints, autobody shops, and print shops. Nitrogen oxides result primarily from high temperature combustion. Significant sources are power plants, industrial furnaces and boilers, and motor vehicles.
How does atmospheric ozone affect human health?
First, it absorbs UV light, reducing human exposure to harmful UV radiation that causes skin cancer and cataracts. Second, when inhaled, it reacts chemically with many biological molecules in the respiratory tract, leading to a number of adverse health effects . This course addresses this second property.
How does ozone affect the atmosphere?
Depending on where it is in the atmosphere, ozone affects life on Earth in either good or bad ways. Stratospheric ozone is formed naturally through the interaction of solar ultraviolet (UV) radiation with molecular oxygen (O2).
When does ozone peak?
Where ozone is formed, peak concentrations usually occur during afternoon hours, when sunlight is the most intense. However, areas downwind of major sources of VOC and NOx may experience ozone peaks in the afternoon and evening, after wind has carried ozone and its VOC and NOx precursors many miles from their sources.
Where is tropospheric ozone found?
Ozone formation is not limited to big cities like Los Angeles, Houston, Atlanta, and New York City. It is also formed in smaller cities like Raleigh, NC and Cincinnati, OH, ...
Does ozone increase in summer?
These reactions have traditionally been viewed as depending upon the presence of heat and sunlight, resulting in higher ambient ozone concentrations in summer months. Within the last decade, however, high ozone concentrations have also been observed under specific circumstances in cold months, where a few high elevation areas in the Western U.S.
What damage can ozone do to the Earth?
Damage to ozone layer can cause penetration of UV rays in the earth surface which damages human organs, plants, etc.
Why does oxygen split?
Oxygen molecule splits into atomic oxygen (O) at higher levels of an atmosphere because of higher energy of UV radiations. Damage to the ozone layer is a cause of concern to us as it forms a layer in the atmosphere which protects us from the harmful UV radiation.
