
Precautions
What are the side effects of salicylic acid? Common side effects of salicylic acid are peeling of the skin, itching, stinging, and change in color of the skin. These are nothing to be worried about. However, serious side effects are: Fast breathing; Difficulty in breathing; Light-headedness, dizziness; Tinnitus; Vomiting, diarrhea, and severe stomach pain
What is the side effect of salicylic acid?
Salicylic acid is a naturally occurring molecule derived from the bark of willow trees. It's part of a class of acids called beta-hydroxy acids, or BHAs, and is known for its ability to help exfoliate and slough away dead skin cells from the skin's surface.
Where does salicylic acid come from?
What are the chemical properties of salicylic acid? Salicylic Acid is a beta hydroxy acid that occurs as a natural compound in plants. It has direct activity as an anti-inflammatory agent and acts as a topical antibacterial agent due to its ability to promote exfoliation.
What are the chemical properties of salicylic acid?
What does salicylic acid do for callus? Salicylic acid helps cause the wart to gradually peel off. This medication is also used to help remove corns and calluses. This product should not be used on the face or on moles, birthmarks, warts with hair growing from them, or genital/anal warts. Salicylic acid is a keratolytic.
What does salicylic acid do to calluses?
What is methyl salicylate used for?
What is the reaction between salicylic acid and acetic anhydride?
What is salicylic acid?
Why is salicylic acid good for hair?
What happens when salicylic acid dissociates?
What are the two methods of preparation of salicylic acid?
What are the physical properties of salicylic acid?
See 4 more
About this website
How is salicylic acid synthesized?
Salicylic acid is produced commercially via the Kolbe-Schmitt process. Here phenol and sodium hydroxide are reacted to make sodium phenoxide. The phenoxide is contacted with CO2 to form sodium salicylate. The salicylate is acidified to give salicylic acid.
How is salicylic acid prepared in the lab?
Place 2.0 g (0.015 mole) of salicylic acid in a 125-mL Erlenmeyer flask. Add 5 mL (0.05 mole) of acetic anhydride, followed by 5 drops of conc. H2SO4 (use a dropper, H2SO4 is highly corrosive) and swirl the flask gently until the salicylic acid dissolves. Heat the flask gently on the steam bath for at least 10 minutes.
Is salicylic acid natural or synthetic?
Natural Occurence Salicylic acid is a naturally occurring compound, which can be isolated from the bark of the willow tree. It can also be synthetically produced, either by biosynthesis of the amino acid phenylalanine, or from phenol.
How many steps are in the synthesis of salicylic acid?
In the first step, hydroxide ion abstracts an acidic proton from phenol to form phenoxide ion. In the next step, phenoxide ion attack C atom of carbon dioxide molecule. Final step is the protonation of carboxylate ion.
Where does salicylic acid come from?
Salicylic acid (2-hydroxybenzoic acid) is a white solid first isolated from the bark of willow trees (Salix spp.), from which it gets its name. It also occurs as the free acid or its esters in many plant species.
Which of the following is used to prepare salicylic acid?
Today the compound is made from dry sodium phenoxide (sodium phenolate) and carbon dioxide, followed by treatment with acid. Most salicylic acid produced commercially is treated with acetic anhydride for the preparation of aspirin.
Is salicylic acid plant based?
Salicylic acid (SA) is a plant hormone which plays a crucial role in the plant defense against various pathogens and abiotic stresses.
Is there a natural form of salicylic acid?
White willow (Salix alba) is a natural source of salicylic acid.
Which natural ingredient has salicylic acid?
Salicylic acid is a colourless crystalline solid. It is a chemical molecule produced in the plant world. It is naturally present in several plants, in particular meadowsweet and willow, a tree known since ancient times, in particular for its anti-inflammatory properties.
How do you make salicylic acid solution?
Shake a small amount of the salicylic acid powder into a bowl. Start with about a tablespoon (13 g) of the powder, and then pour in about 1 teaspoon (5 mL) of the propylene glycol. Whisk the two ingredients together until they're combined in a paste. Try to achieve the consistency of toothpaste.
What type of reaction is the synthesis of aspirin from salicylic acid?
acetylationAspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride.
How do you synthesize aspirin with salicylic acid?
To prepare aspirin, salicylic acid is reacted with an excess of acetic anhydride. A small amount of a strong acid is used as a catalyst which speeds up the reaction. In this experiment, sulfuric acid will be used as the catalyst. The excess acetic anhydride will be quenched (reacted) with the addition of water.
How do you make salicylic acid solution?
A kind of preparation method of salicylic acid solution comprises: dissolve pyrrolidone sodium carboxylate in water, preparation pyrrolidone sodium carboxylate aqueous solution; Predissolve salicylic acid in the pyrrolidone sodium carboxylate aqueous solution, or in ethanol the predissolve salicylic acid; Add ...
How do you make salicylic acid from sodium salicylate?
A typical operation would be to dilute the crude salts to about 1 ton sodium salicylate in 8 to 10 tons of water and precipitate the salicylic acid by adding sulfuric acid at 50-60°C. This would give a salicylic acid of 99.5% purity in a yield of about 85% of the salicylic acid present.
Which method is used to prepare salicylic acid from phenol?
General Method for Synthesis of Salicylic Acids from Phenols via Pd-Catalyzed Silanol-Directed C–H Carboxylation.
How is aspirin made in a lab?
Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. The molecular weight of aspirin is 180.16g/mol. It is odourless, colourless to white crystals or crystalline powder.
Salicylic Acid: Uses, Structure, Properties and Methods ... - Collegedunia
Methods of Preparation of Salicylic Acid Salicylic acid is prepared generally in two ways. These are: Kolbe-Schmitt process: This method is used to produce salicylic acid commercially.It is a carboxylation chemical reaction in which at first sodium phenoxide is formed by reacting phenol and sodium hydroxide.
What is the best way to induce vomiting?
INHALATION: move to fresh air. INGESTION: induce vomiting and get medical attention promptly. EYES: promptly flush with water for 15 min. and get medical attention. SKIN: wash with soap and water. (USCG, 1999)
What are the hazards of combustion products?
Special Hazards of Combustion Products: Irritating vapors of unburned material and phenol may form in fire. Behavior in Fire: Sublimes and forms vapor or dust that may explode (USCG, 1999)
What foods contain salicylic acid?
Salicylic acid has been quantitatively detected in many foods, including whole tomatoes, cinnamon, oregano, thyme, red wine, beer, honey, licorice, and raisins (2). A salicylic acid concentration of 1474.7 mg/100 g was measured in cumin, while other common spices contained up to 23.3 mg/100 g salicylic acid (3). Salicylic acid has also been detected in the blueberry Vaccinium arctostaphylos (4) and barley (5). A survey of vegetable soup found that the organic soup tested had a median salicylic acid concentration of 117 ng/g salicylic acid, while the non-organic soup had a median concentration of 20 ng/g (6).
What is the koc of salicylic acid?
The Koc of salicylic acid is estimated as 404 (SRC), using a log Kow of 2.26 (1) and a regression-derived equation (2). According to a classification scheme (3), this estimated Koc value suggests that salicylic acid is expected to have moderate mobility in soil. The pKa of salicylic acid is 2.98 (4), indicating that this compound will primarily exist in anion form in the environment and anions generally do not adsorb more strongly to soils containing organic carbon and clay than their neutral counterparts (5).
How much salicylic acid is in Dutch food?
Using food intake data from the Dutch Food Consumption Survey and measurements of salicylic acid in foods, the average daily intake of salicylic acid has been estimated to be 0-5 mg/day (1).
What is SOCMI in the chemical industry?
This action promulgates standards of performance for equipment leaks of Volatile Organic Compounds (VOC) in the Synthetic Organic Chemical Manufacturing Industry (SOCMI). The intended effect of these standards is to require all newly constructed, modified, and reconstructed SOCMI process units to use the best demonstrated system of continuous emission reduction for equipment leaks of VOC, considering costs, non air quality health and environmental impact and energy requirements. Salicylic acid is produced, as an intermediate or a final product, by process units covered under this subpart.
How does salicylic acid help with acne?
Salicylic acid treats acne by causing skin cells to slough off more readily, preventing pores from clogging up. This effect on skin cells also makes salicylic acid an active ingredient in several shampoos meant to treat dandruff. Use of straight salicylic solution may cause hyperpigmentation on unpretreated skin for those with darker skin types (Fitzpatrick phototypes IV, V, VI), as well as with the lack of use of a broad spectrum sunblock. Subsalicylate in combination with bismuth form the popular stomach relief aid known commonly as Pepto-Bismol. When combined the two key ingredients help control diarrhea, nausea, heartburn, and even gas. It is also very mildly anti-biotic.
What is the role of salicylic acid in plants?
Salicylic acid is a phenolic phytohormone, and is found in plants with roles in plant growth and development, photosynthesis, transpiration, and ion uptake and transport. Salicylic acid is involved in endogenous signaling, mediating plant defense against pathogens.
What is white willow bark?
White willow ( Salix alba) is a natural source of salicylic acid. Hippocrates, Galen, Pliny the Elder, and others knew that willow bark could ease pain and reduce fevers. It was used in Europe and China to treat these conditions. This remedy is mentioned in texts from Ancient Egypt, Sumer, and Assyria.
What acid is used to make salicylic acid?
Acidification of the product with sulfuric acid gives salicylic acid: It can also be prepared by the hydrolysis of aspirin (acetylsalicylic acid) or methyl salicylate (oil of wintergreen) with a strong acid or base.
What is the active ingredient in Pepto Bismol?
Bismuth subsalicylate, a salt of bismuth and salicylic acid, is the active ingredient in stomach-relief aids such as Pepto-Bismol, is the main ingredient of Kaopectate, and "displays anti-inflammatory action (due to salicylic acid) and also acts as an antacid and mild antibiotic". Other derivatives include methyl salicylate used as a liniment ...
What is the name of the acid that is used to make aspirin?
Aspirin (acetylsalicylic acid or ASA) is prepared by the esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride .
How is salicylate prepared?
Sodium salicylate is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (115 °C) – a method known as the Kolbe-Schmitt reaction. Acidification of the product with sulfuric acid gives salicylic acid:
What is the chemical formula for salicylic acid?
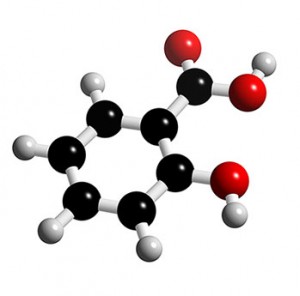
Chemical compound. Salicylic acid is an organic compound with the formula HOC 6 H 4 CO 2 H. A colorless solid, it is a precursor to and a metabolite of aspirin (acetylsalicylic acid). It is a plant hormone. The name is from Latin salix for willow tree.
What is salicylic acid esterified with?
Salicylic acid esterified with methanol in the presence of an acid catalyst gives methyl salicylate, synthetic oil of wintergreen, which is used as a flavouring agent. Treatment of salicylic acid with phenol gives phenyl salicylate, which is used for sunburn creams and enteric-coated pills and to make salicylanilide for use as a fungicide ...
What temperature does salicylic acid melt?
Pure salicylic acid crystallizes from hot water in the form of white needles, which sublime without decomposition at temperatures up to 155 °C (311 °F) and melt at 159 °C (318 °F). Above 200 °C (392 °F), the acid decomposes to phenol and carbon dioxide.
What is the name of the acid that is used in preparation of aspirin?
Salicylic acid, also called ortho-hydroxybenzoic acid, a white, crystalline solid that is used chiefly in the preparation of aspirin and other pharmaceutical products. The free acid occurs naturally in small amounts in many plants, particularly the various species of Spiraea. The methyl ester also occurs widely in nature;
What is salicylic acid?
chemical compound. Emeritus Professor of Chemistry, Beloit College, Wisconsin. Salicylic acid, also called ortho-hydroxybenzoic acid, a white, crystalline solid that is used chiefly in the preparation of aspirin and other pharmaceutical products. The free acid occurs naturally in small amounts in many plants, particularly the various species ...
Is salicylic acid a carboxylic acid?
Salicylic acid is both a carboxylic acid and a phenol, so it can be esterified in two ways, with both giving rise to familiar products. In methyl salicylate (oil of wintergreen), the COOH group of salicylic acidis esterified with methanol (CH3OH), whereas in acetylsalicylic…
Who discovered salicylic acid?
Salicylic acid was first prepared by the Italian chemist Raffaele Piria in 1838 from salicylaldehyde. In 1860 the German chemists Hermann Kolbe and Eduard Lautemann discovered a synthesis based on phenol and carbon dioxide. Today the compound is made from dry sodium phenoxide (sodium phenolate) and carbon dioxide, followed by treatment with acid.
How long does it take for aspirin to excrete?
It takes about 48 hours to excrete an aspirin completely. The half-life of aspirin in the blood stream is 13-19 minutes and the half-life of its metabolite salicylate is around 3.5-4.5 hours. Aspirin’s inhibition of COX-1 results in reduced platelet aggregation for the 7-10-day average lifespan of platelets 1.
How do prostaglandins work?
Prostaglandins are found throughout the body and are made to help manage injury or infection. Prostaglandins upregulate the sensitivity of pain receptors. As a control mechanism, they act locally at the site of synthesis which limits the extent of their activity. They are also broken down rapidly by the body.
How is aspirin made?
Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. The molecular weight of aspirin is 180.16g/mol. It is odourless, colourless to white crystals or crystalline powder. Aspirin is an oral non-steroidal anti-inflammatory drug (NSAID) that is rapidly absorbed from the stomach and ...
Does aspirin increase platelet aggregation?
From a cardiovascular perspective aspirin also has an important role: Thromboxane A2 (TXA2) is a lipid that stimulates new platelet formation and increases platelet aggregation . Aspirin inhibits the production of thromboxane A2 (TXA2) by stopping the conversion of arachidonic acid to TXA2.
Which enzymes produce prostaglandins?
The enzymes that produce prostaglandins are cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), they have diverse roles and are widely dispersed throughout body tissue. Cox-1 has a protective role for the stomach lining and COX-2 is involved in pain and inflammation. Aspirin binds to and acetylates serine (an amino acid used by ...
Does aspirin help with myocardial infarction?
In this way aspirin can help lower the risk of future myocardial infarction ( MI) or stroke 1,3. Aspirin, therefore, has an analgesic (reduces pain), anti-inflammatory (reduces redness and swelling), anti-platelet (reduces blood clots) and antipyretic (temperature reduction) effects 1,2,3. In cancer, aspirin is believed to impact a number ...
Does aspirin help with thrombus formation?
This aspirin effect is mediated via COX-1 inhibition within platelets and helps stop the platelets from sticking to each other or to plaques within the artery therefore reducing the risk of blood clot (thrombus) formation within the blood stream. In this way aspirin can help lower the risk of future myocardial infarction (MI) or stroke 1,3.
Amount of Reactant Needed to Produce a Product
Todd Helmenstine is a science writer and illustrator who has taught physics and math at the college level. He holds bachelor's degrees in both physics and mathematics.
Problem
Aspirin is prepared from the reaction of salicylic acid (C 7 H 6 O 3) and acetic anhydride (C 4 H 6 O 3) to produce aspirin (C 9 H 8 O 4) and acetic acid (HC 2 H 3 O 2 ). The formula for this reaction is
Answer
766.67 grams of salicylic acid are needed to produce 1000 1-gram aspirin tablets.
What is methyl salicylate used for?
It can be used for the preparation of salicylic acid. In this reaction, methyl salicylate is reacted with sodium hydroxide to lead to the formation of a sodium salt intermediate of salicylic acid, named disodium salicylate, which upon undergoing further reaction with sulphuric acid leads to the formation of salicylic acid.
What is the reaction between salicylic acid and acetic anhydride?
In this reaction, salicylic acid is reacted with acetic anhydride in an acidic medium which leads to the acetylation of the hydroxyl group present in the salicylic acid, thereby resulting in the production of acetylsalicylic acid (aspirin). Acetic acid is produced as a by-product of this reaction, which is also present as one ...
What is salicylic acid?
VIEW MORE. Salicylic Acid is a small aromatic acid whose chemical name is monohydroxybenzoic acid. It is lipophilic in nature. It was first derived from the bark of Willow Tree. It derives its common name from a variety of sources related to it with a similar name, e.g., it is derived as a metabolic product of salicin ...
Why is salicylic acid good for hair?
It is because salicylic acid prevents the deposition of sebum in the skin pores and around hair follicles. It helps in clearing away the dead and flaky skin cells from your scalp, thereby preventing the occurrence of dandruff.
What happens when salicylic acid dissociates?
In aqueous solution, Salicylic Acid, being an organic acid, dissociates to lose a proton from the carboxylic acid functional group. The resulting carboxylate ion (–COO –) undergoes intermolecular interaction with the hydrogen atom of the hydroxyl group (–OH), thereby leading to the formation of an intramolecular hydrogen bond.
What are the two methods of preparation of salicylic acid?
Methods of Preparation of Salicylic Acid. There are two most commonly used methods for the preparation of salicylic acid. These methods are discussed below: • From Phenol: When phenol is reacted with sodium hydroxide, it forms sodium phenoxide which is then further allowed to undergo distillation and dehydration.
What are the physical properties of salicylic acid?
Physical Properties of Salicylic Acid. • Salicylic Acid exist as clear white or colourless and odourless needle-shaped crystals at room temperature. • Salicylic Acid contains two hydrogen bond donors and three hydrogen bond acceptors in its molecule.

Overview
Salicylic acid is used on the skin to treat psoriasis and other dry, scaly skin conditions. This medication is also used to help remove dead skin from warts, the palms of the hands, and the soles of the feet.
May Treat: Acne vulgaris · Calluses and corns · Dandruff · Hyperkeratosis · Keratoderma and more
Brand Names: Salex · Virasal · Bensal HP · Salvax · Keralyt and more
Drug Class: Acne Therapy Topical - Keratolytic · Dermatological - Keratolytic-Antimitotic Single Agents
Availability: Prescription sometimes needed
Pregnancy: Consult a doctor before using
Lactation: Consult a doctor before using
Production and chemical reactions
Uses
Mechanism of action
Safety
History
Salicylic acid is biosynthesized from the amino acid phenylalanine. In Arabidopsis thaliana, it can be synthesized via a phenylalanine-independent pathway.
Sodium salicylate is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (115 °C) – a method known as the Kolbe-Schmitt reaction. Acidifi…
Dietary sources
Salicylic acid as a medication is commonly used to remove the outer layer of the skin. As such, it is used to treat warts, psoriasis, acne vulgaris, ringworm, dandruff, and ichthyosis.
Similar to other hydroxy acids, salicylic acid is an ingredient in many skincare products for the treatment of seborrhoeic dermatitis, acne, psoriasis, calluses, c…
Plant hormone
Salicylic acid modulates COX-1 enzymatic activity to decrease the formation of pro-inflammatory prostaglandins. Salicylate may competitively inhibit prostaglandin formation. Salicylate's antirheumatic (nonsteroidal anti-inflammatory) actions are a result of its analgesic and anti-inflammatory mechanisms.
Salicylic acid works by causing the cells of the epidermis to slough off more readily, preventing …