How is the mass number of an isotope expressed in the name of an atom? The mass number of an isotope is indicated in the name of the isotope by writing the name of the element followed by a hyphen and the mass number. Examples include carbon-14, uranium-235, and oxygen-17.
How do you calculate the atomic mass of isotopes?
How is the mass number of an isotope expressed in the name of an atom? In the isotope symbol of each atom, there is a superscripted (raised) number. This number is also used in the name of the atom (i.e., carbon-12). It is called the mass number. THE ATOM BECAUSE THEY EACH HAVE A MASS OF 1 AMU. Click to see full answer.
What is the average atomic mass of an isotope?
Nov 10, 2016 · How is the mass number of an isotope expressed in the name of an atom? - Answers The mass number is the sum of protons and neutrons in the atomic nucleus. If you think to a name as uranium-238 the...
What is the definition of atomic mass and atomic number?
The atomic mass is expressed in unified atomic mass units (u). Some of the atoms contain the same number of protons but a different mass number due to a different number of neutrons. They are called isotopes. For example; three isotopes of …
What are the isotopes of an atom?
Apr 05, 2022 · It is expressed in atomic mass units. The letter 'A' is used to signify it. Mass Number The mass number is different for the isotopes of the same element. As a result, the number of neutrons (N) in a given nucleus is determined by the difference between the mass number and the atomic number Z: N = A Z = N = A Z = N = A Z = N = A Z = N

How are isotopes indicated when written?
Isotope Notation Isotopes are notated in multiple ways. Most commonly, they are specified by the name or symbol of the particular element, immediately following by a hyphen and the mass number (e.g., carbon-14 or C-14).
Is the mass number the mass of an isotope?
Mass Number is the number of protons and neutrons in an isotope. This is a whole number. We use the mass number in naming isotopes, like Carbon-12 or Oxygen-17. Atomic Mass is the mass of the entire atom of an isotope.
Is an isotope identified by its mass number?
An isotope and/or nuclide is specified by the name of the particular element (this indicates the atomic number) followed by a hyphen and the mass number (e.g. helium-3, helium-4, carbon-12, carbon-14, uranium-235 and uranium-239).
How do you find the mass of an isotope?
For any given isotope, the sum of the numbers of protons and neutrons in the nucleus is called the mass number. This is because each proton and each neutron weigh one atomic mass unit (amu). By adding together the number of protons and neutrons and multiplying by 1 amu, you can calculate the mass of the atom.
What is the mass of this isotope?
The mass number of an isotope is the total number of protons and neutrons in an atomic nucleus. If you know that a nucleus has 6 protons and 6 neutrons, then its mass number is 12. If the nucleus has 6 protons and 7 neutrons, then its mass number is 13.
What is Isobar Class 9?
Isobars are defined as. The atoms that have same number of nucleons. Isobars of different chemical elements have different atomic number but have the same mass number.Sep 15, 2021
How do you find the number of isotopes?
Subtract the atomic number (the number of protons) from the rounded atomic weight. This gives you the number of neutrons in the most common isotope. Use the interactive periodic table at The Berkeley Laboratory Isotopes Project to find what other isotopes of that element exist.Oct 25, 2017
Why is mass number different in isotopes?
Basic principles. Isotopes are atoms of the same element that have different numbers of neutrons but the same number of protons and electrons. The difference in the number of neutrons between the various isotopes of an element means that the various isotopes have different masses.
What is the relative isotopic mass?
The relative isotopic mass is a unitless quantity with respect to some standard mass quantity. The relative atomic mass can be taken as the weighted mean mass of an atom of an element compared to the mass of 1/12 of the mass of an atom in C-12.
Why are isotopes different?
We know that isotopes are atoms with the same atomic number but different mass numbers due to a different number of neutrons. The mass number of an element is a whole number whereas the actual mass of an atom is not a whole number except for carbon-12. For example, the atomic mass of Lithium is 6.941 Da.
What is the total number of protons in an atom?
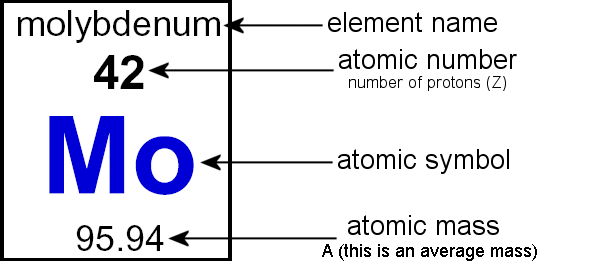
The total number of protons in an atom is called the atomic number. The sum of the number of neutrons and protons is known as mass number. In an atom, the number of protons is always equal to the total number of electrons which makes it neutral due to equal and opposite charges of electrons and protons. The atomic mass is expressed in unified ...
Why are isotopes useful?
Some of the isotopes are very useful and widely used in various fields like medical and chemical industries. Isotopes have the same chemical properties as they have the same number of electrons and their arrangement in the shell. They have a different number of neutrons which affects their mass and mass number.
Do isotopes have the same mass?
They have the same atomic number but different mass number; 1, 2 and 3 respectively. Isotopes are found in different percentage in nature. Some of the isotopes are found in abundance whereas some of them are radioactive and decay continually in nature.
