The atomic
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion (thermonuclear weapon). Both reactions release vast quantities of energy from relatively small amounts of matter.
What is the formula to find the atomic weight?
atomic weight = mass a x fract a + mass b x fract b If there were three isotopes, you would add a 'c' entry. If there were four isotopes, you'd add a 'd', etc.
What determines the atomic weight of an atom?
- every atom is composed of a nucleus and one or more electrons.
- the nucleus is made of one or more protons and tipically the same number of neutrons.
- the electrons are usually the same number of protons.
- the mass of the atom is determined at 99.95% by the nucleus (electrons are very small)
How to calculate the atomic weight?
Locate atomic mass on the periodic table.
- Note that the relative atomic masses listed on the periodic table are average values for the associated element. ...
- Relative atomic masses, as listed on the periodic table, are used to calculate molar masses for atoms and molecules. ...
- For example, the atomic mass of iron is 55.847 amu, which means one mole of iron atoms would weigh 55.847 grams.
What is average atomic mass and how is it calculated?
What is average atomic mass and how is it calculated? The average atomic mass for an element is calculated by summing the masses of the element’s isotopes, each multiplied by its natural abundance on Earth. When doing any mass calculations involving elements or compounds, always use average atomic mass, which can be found on the periodic table.
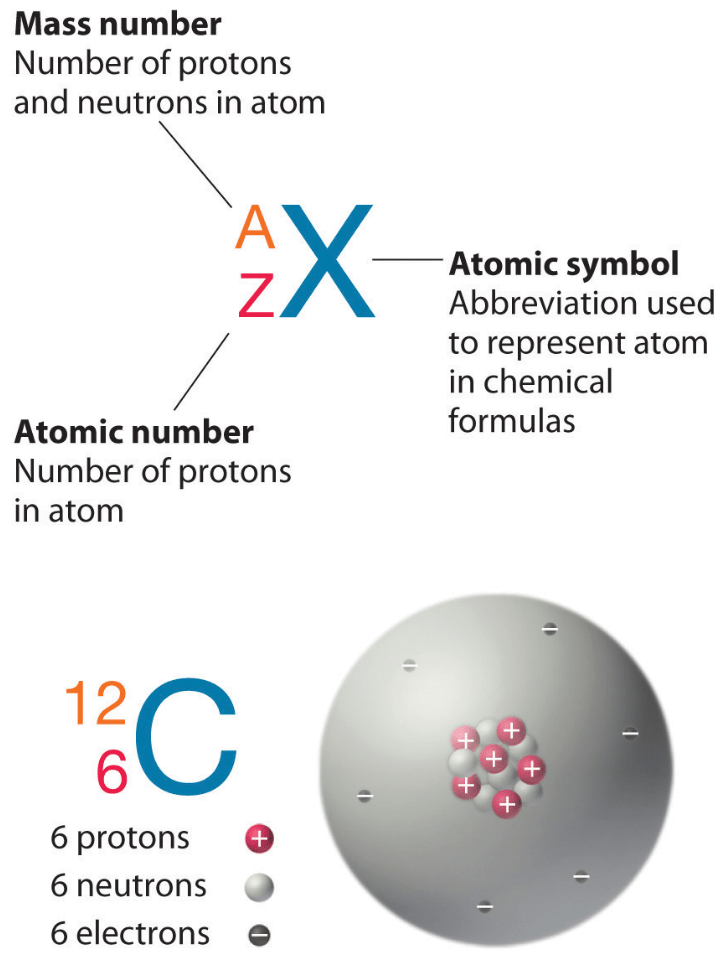
How do you find the weight of an atom?
The atomic weight of any atom can be found by multiplying the abundance of an isotope of an element by the atomic mass of the element and then adding the results together. This equation can be used with elements with two or more isotopes: Carbon-12: 0.9889 x 12.0000 = 11.8668.
What is atomic weight and how it is calculated?
The atomic weight is calculated by adding the mass of each isotope multiplied by its fractional abundance. For example, for an element with 2 isotopes: atomic weight = massa x fracta + massb x fractb. If there were three isotopes, you would add a 'c' entry.
How is atomic weight determined quizlet?
A) Atomic weight is determined by the number of electrons in an atom of a given element.
What is the easiest way to determine atomic weight?
To calculate the atomic mass of a single atom of an element, add up the mass of protons and neutrons. Example: Find the atomic mass of an isotope of carbon that has 7 neutrons. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.
What is atomic weight measured in?
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope.
What is difference between atomic mass and atomic weight?
Atomic mass represents the mass of a single isotope or single atom. Atomic weight can be defined as the average weight of an element with respect to all its isotopes and their relative abundances.
What is true about atomic weight quizlet?
Which is true concerning atomic weight? Atomic weight is the average of the mass numbers of all the isotopes of an element.
What does atomic weight tell us quizlet?
The atomic mass tells us the weight of protons and neutrons.
What is the atomic mass quizlet?
What is atomic mass? the sum of all protons and neutrons in the atomic nucleus.
Do we have to memorize atomic mass?
question_answer Answers(4) For your grade you need to remember atomic masses of first 20 elements. The atomic mass is double the atomic number of an element. But it is not applicable for all the elements in the periodic table.
What is the easiest way to remember the first 20 elements?
Silly Patrick Stays Close. Arthur Kisses Carrie. Here He Lies Beneath Bed Clothes, Nothing On, Feeling Nervous, Naughty Margret Always Sighs, ” Please Stop Clowning Around ” (18 elements)...Hydrogen -H.Helium -He.Lithium -Li.Beryllium -Be.Boron -B.Carbon -C.Nitrogen -N.Oxygen -O.More items...
How is the atomic mass of carbon or any other element determined?
1 Answer. The atomic mass of carbon, or any other element is determined By adding the number of protons and the number of neutrons.
How do you calculate atomic number?
The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. For example, in a sodium atom, there are 11 electrons and 11 protons. Thus the atomic number of Na atom = number of electrons = number of protons = 11.
How do you calculate the atomic mass of Class 9?
Mathematically, mass of one atom of an element = atomic mass X (1/12th) of the mass of one atom of carbon. atomic mass = mass of one atom of an element / (1/12th) of the mass of one atom of carbon. Easy way to calculate atomic mass of oxygen atom.
What is atomic mass Class 11?
Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons.
How to find the atomic weight of an element?
The atomic weight of an element depends on the abundance of its isotopes. If you know the mass of the isotopes and the fractional abundance of the isotopes, you can calculate the element's atomic weight in atomic mass units (expressed as u, Da, or amu). The atomic weight is calculated by adding the mass of each isotope multiplied by its fractional ...
How to calculate atomic weight?
The atomic weight is calculated by adding the mass of each isotope multiplied by its fractional abundance. For example, for an element with 2 isotopes: If there were three isotopes, you would add a 'c' entry. If there were four isotopes, you'd add a 'd', etc.
What would you do if there were 3 isotopes?
If there were three isotopes, you would add a 'c' entry. If there were four isotopes, you'd add a 'd', etc.
Why the One-Percent Difference?
As to why there is a difference? There are a number of reasons for it. We rounded off Avogadro’s number down. We rounded the weights of the subatomic particles.
How to find the atomic weight of gold?
Let’s pick an element out of the hat. Let’s take gold (If we can’t afford it, at least we can talk about it.). To calculate atom weight by method two, we need to look at the Periodic Table of the Elements. There we see gold, chemical symbol Au, as element number 79. At the bottom of the little square, we see the number 196.97. This is the atomic weight of gold. The atomic weight in grams of an element equals the weight of an Avogadro’s number of that element. Huh? What?
What is an isotope?
An isotope is an element that has the same number of protons and electrons as any other atom of an element, but a differing number of neutrons. What makes it more difficult is that percentages of isotopes found in nature vary for different elements.
How many protons does gold have?
Gold found in nature has 79 protons, 79 electrons, and 118 neutrons. The weight of a proton in grams is 1.673 x 10 -24 grams. The weight of a neutron is 1.675 x 10 -24 grams. The weight of an electron is 1/1836th the weight of a proton, so it weighs 9.11 x 10 -28 grams. This gives us,
How to find the weight of energy?
The mass, or for our purposes, the weight of energy can be found by changing this around to, m = E/c2. This weight (actually, mass) is added to the weight (mass) of an atom. The combination of adjustments should put ...
What is the Nav of a gas?
A constant was derived, which was later named in his honor. That number is currently placed at. Nav = 6.022 x 1023 moles-1.
Is the weight of an atom equal to the weight of all its subatomic particles?
Also, the weight of an atom is not strictly equal to the weight of all its subatomic particles. The reader is doubtless well aware that atomic fission is sometimes called “atom splitting.”. The energy given off by the splitting of an atom is tremendous. Curiously, the energy weighs something.
Why does the atomic weight of an element increase?
As we can see from the above graph, initially for lighter elements, the atomic weight is almost twice the atomic number. For heavier elements, as the atomic number increases, the atomic weight exceeds twice the atomic number. This divergence in the graph is due to an increase in the neutron number. For heavier elements, the number of neutrons in the nucleus is more than the number of protons which increases the mass of an atom.
What is the atomic weight of an atom?
The atomic weight (also known as relative atomic mass) is a quantity used to express the average weight of an atom. Atoms consist of electrons, protons, and neutrons. Protons and neutrons are mainly responsible for the mass of an atom. For a given element, the proton number (more commonly known as the atomic number) is fixed, but the neutron number can vary. Such elements are called isotopes. Because of this variance in the neutron number, an atom of the same element can have a different atomic mass.
What is the atomic mass of chlorine?
Consider an example of chlorine, which has two naturally-occurring isotopes: 35 Cl and 37 Cl. The atomic mass of these isotopes is 34.969 u and 36.966 u respectively. The dilemma here is which atomic mass to consider. To solve this problem and have better accuracy in calculations, we average out atomic masses. This average atomic mass is called the atomic weight.
How many isotopes does chlorine have?
Figure 1: Chlorine has two naturally-occurring isotopes: 35 Cl, 75.76 %; 37 Cl, 24.24 %. The weighted average of their atomic masses gives the atomic weight of chlorine.
How to find molecular weight?
The molecular weight of any molecule can be determined by knowing its molecular formula and atomic weights of its elements. It is synonymous with the relative molar mass.
How to calculate atomic weight?
The atomic weight of any element can be calculated by knowing the individual atomic mass and the relative abundance of isotopes.
What is the unit used to express the atomic mass?
The dalton or the unified mass unit (u) or the atomic mass unit (amu) is the unit used to express the atomic mass. The atomic weight is more practical and often used by chemists. It is used when we are dealing with an isotopic atom. It is more likely used by physicists.
Why were absolute weights of atoms not determined?
According to the Science Encyclopedia, Because atoms were much too small to be seen or measured by any common methods, absolute weights of atoms could not be determined. Rather, these first measurements were made by comparing weights of various atoms to hydrogen.
What does Gay-Lussac say about gases?
Gay-Lussac later in early 1800s mentioned that gases react with each other in certain proportions. "For example, at the same temperature and pressure, two volumes of hydrogen react with one volume of oxygen and form two volumes of water.". [1].
What is the meaning of "back up"?
Making statements based on opinion; back them up with references or personal experience.
Who came up with the long form of the periodic table?
Mendeleev later came up with periodic classification based on his systematic understanding and analysis of the then known elements and classified them in the periodic table. This later led to coming up with the long form of periodic table which is currently in use.
Who was the first to explain the law of mass conservation during chemical reactions?
These three chemists contributed to a great extent to the understanding of absolute formulas of substances. Lomonosov earlier was the first to come up to explain four types of chemical reactions in mid of 1700s and was the one to explain law of mass conservation during chemical reactions.
What is the atomic number of an atom?
Atomic number is equal to the number of neutrons in an atom's nucleus.
What is the difference between potential and kinetic energy?
A) Kinetic energy is stored energy and has the capacity to do work; potential energy is expressed through motion.
What is the atomic weight of an element?
C) Atomic weight of an element is approximately equal to the mass number of its most abundant isotope.
Why do cells need to produce large amounts of enzymes?
A) Because their catalytic role renders most enzymes ineffective after their initial activity, a cell must produce large amounts of each enzyme in order to perform effectively.
Is a mixture homogeneous or heterogeneous?
A) Some mixtures are homogenous, while others are heterogeneous. All compounds are homogeneous.