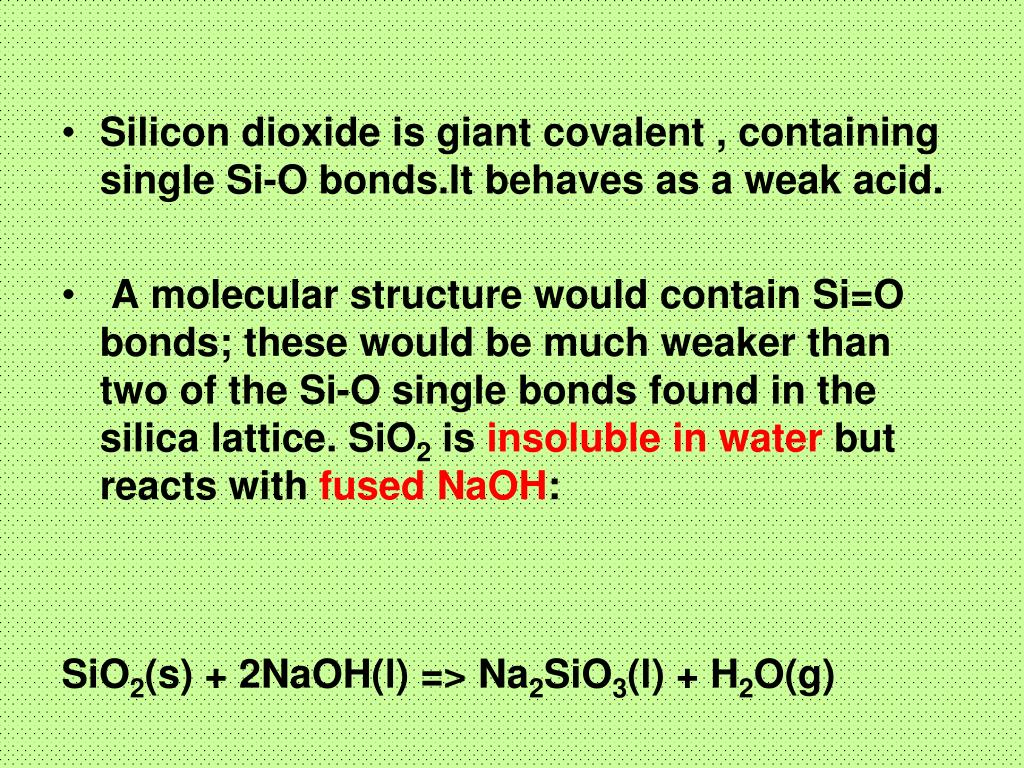
Sodium Oxide having a chemical formula of Na2O, is a metal oxide. It is also known as an alkali metal oxide as it comprises two sodium and one Oxygen atoms. The compound is the base anhydride for NaOH, as when Na2O reacts with water, it produces NaOH.
What is the formula of Na2O?
Na₂OSodium oxide / Formula
How many atoms are represented in Na?
11We know that the atomic number of sodium is 11.
What charge is Na2O?
03.1Computed PropertiesProperty NameProperty ValueReferenceFormal Charge0Computed by PubChemComplexity0Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)Isotope Atom Count0Computed by PubChemDefined Atom Stereocenter Count0Computed by PubChem13 more rows
How many atoms are in 3 moles Na?
Hence, 3 moles of sodium contains 1.8066 x 10^24 atoms and has a mass equal to 69 grams.
How many electrons are there in Na+?
10As Sodium loses its electron it forms a Sodium ion and the total number of electrons are 10.
How many ions are in Na2O?
Sodium oxide molecules are made up of two sodium cations and one oxygen anion. Thus, Na2O molecules feature two sodium-oxygen ionic bonds.
How many lone pairs does Na2O have?
Two Na atoms are present at the terminal position and O is present at the central in Na2O. there are two single bond present and two pairs of lone pairs over the O atom only.
What ions are in Na2O?
The ions present in Na2O are Na+ and O2− ions.
How many atoms are present in H₂so₄?
Sulfuric acid is a strong mineral acid that is soluble in water at all concentrations. Sulfuric acid contains 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms.
How many elements are in an atom?
There are 118 elements, but only 92 occur naturally. The remaining elements have only been made in laboratories and are unstable. Each element is designated by its chemical symbol, which is a single capital letter or, when the first letter is already “taken” by another element, a combination of two letters.
How many atoms of sodium are there in the formula for table salt nacl?
0:081:15How to Find the Number of Atoms in NaCl (Sodium chloride) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo we assume that just to be a one so there's one n a in nacl.MoreSo we assume that just to be a one so there's one n a in nacl.
How many atoms of sodium and oxygen respectively are in a particle of sodium oxide Na2O?
Structure of Sodium oxide-Na2O Molecules Sodium oxide molecules are made up of two sodium cations and one oxygen anion.
How many ionic bonds does Na2O have?
Sodium oxide molecules are made up of two sodium cations and one oxygen anion. Thus, Na2O molecules feature two sodium-oxygen ionic bonds. The structure of sodium oxide molecules is illustrated below.
What is Na2O used for?
Uses of Sodium Oxide – Na2O. It is an active flux and used to create glazes which give exciting colour responses. The more notable responses are blue from copper oxide and purple from cobalt and manganese oxides. Used in bodies except where it has been introduced primarily as a deflocculant for casting slips.
What is Sodium Oxide?
Sodium oxide is an alkali metal oxide with the chemical formula Na 2 O.
What is the reaction of sodium oxide and carbon dioxide?
Sodium oxide reacts with carbon dioxide to form sodium carbonate. The chemical equation is given below. Sodium oxide reacts with some acids (such as hydrochloric acid) to form sodium chloride and water. The chemical equation for this reaction is given below.
What is the name of the app that teaches you about sodium oxide?
To learn more about sodium oxide and other important chemical compounds containing sodium (such as sodium bicarbonate ), download BYJU’S – The Learning App.
How is sodium oxide produced?
Sodium oxide can be produced by reacting metallic sodium with sodium hydroxide. The chemical equation for this reaction is:
What is the name of the white powder that forms when sodium ions combine with oxygen?
It forms a white powder called sodium oxide. Within the earth’s crust, sodium ions often combine with the oxygen in soil and rocks to form sodium oxide (Na 2 O), one of the most common forms of sodium.
Answer
As a product you have 2Na2 = 4 atoms Na. The substances sodium (Na), oxygen (O2), and sodium oxide (Na2O) participate in this chemical reaction: 4Na + O2 → 2Na2O. "Mass can be neither created nor destroyed", therefore you can't have more mass on one side than another.
Answer
The substances sodium (Na), oxygen (O2), and sodium oxide (Na2O) participate in this chemical reaction
New questions in Chemistry
One side of a rectangular field is 15 metres. The length of diagonal of this rectangular field is17 metres. Find the area of this rectangular field.