
How many electrons are in the outermost shell of sodium?
The outermost shell of the sodium ion is the second electron shell, which has eight electrons in it. What is the number of electrons gained or lost in chlorine? Since it has 1 more proton than electrons, sodium has a charge of +1, making it a positive ion. Chlorine gains an electron, leaving it with 17 protons and 18 electrons.
How many protons and neutrons does sodium have?
The number of neutrons is equal to the Atomic Mass minus the Atomic Number. So for your question, the Periodic Table tells us that sodium has an Atomic Number of 11, so there are 11 protons and 11 electrons. The Periodic Table tells us that sodium has an Atomic Mass of ≈23. So there are 23 - 11 = 12 neutrons.
How to determine the number of electron shells?
Thus, group number is a good predictor of how reactive each element will be:
- Helium ( ), neon ( ), and argon ( ), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. ...
- Hydrogen ( ), lithium ( ), and sodium ( ), as group 1 elements, have just one electron in their outermost shells. ...
- Fluorine () and chlorine ( ), as group 17 elements, have seven electrons in their outermost shells. ...
How many electrons are in each shell including 3p orbitals?
The p sublevel has 3 orbitals, so can contain 6 electrons max. The d sublevel has 5 orbitals, so can contain 10 electrons max. What is the maximum number of electrons in the first orbit? Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on.
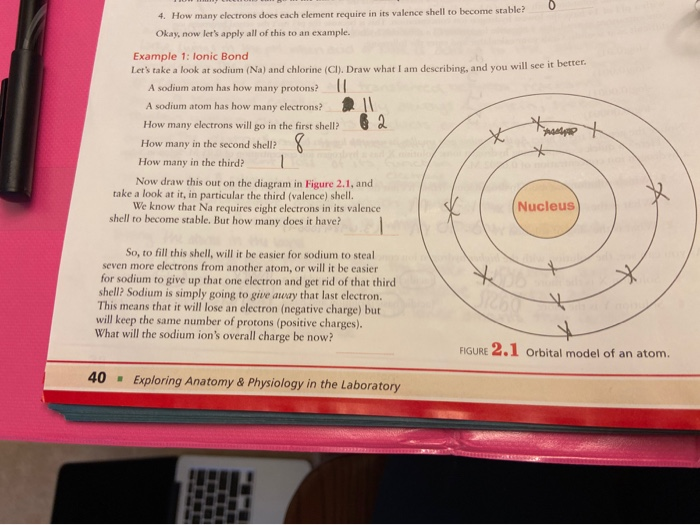
How many electrons does sodium have in its first shell?
two electronsSo... for the element of SODIUM, you already know that the atomic number tells you the number of electrons. That means there are 11 electrons in a sodium atom. Looking at the picture, you can see there are two electrons in shell one, eight in shell two, and only one in shell three.
How many electrons will go in the second shell of sodium?
eight electronsThe outermost shell of the sodium ion is the second electron shell, which has eight electrons in it.
How many electrons shells does sodium have?
The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus....List of elements with electrons per shell.Z11ElementSodiumNo. of electrons/shell2, 8, 1Group169 more columns
How many electrons will go in the third shell of sodium?
There is only one electron in the third shell of a neutral sodium atom.
How many electrons does sodium gain or lose?
Tell students that when an atom gains or loses an electron, it becomes an ion. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Since it has 1 more proton than electrons, sodium has a charge of +1, making it a positive ion.
How many electrons will sodium lose?
Forming positive ions The atom is more stable if it has a full outer shell. A sodium atom can lose its outer electron. It will still have 11 positive protons but only 10 negative electrons.
How many electrons go in each shell?
The first shell (closest to the nucleus) can hold two electrons. The second shell can hold 8 electrons. The third shell can hold 32 electrons. Within the shells, electrons are further grouped into subshells of four different types, identified as s, p, d, and f in order of increasing energy.
Does sodium have 1 valence electrons?
A: An atom of a group 1 element such as sodium has just one valence electron.
How many electrons would an Na+ ion have in its outermost shell?
eight electronsThe positive charge dictates all the properties of Na+ , since otherwise it is stable and inert with its eight electrons in the valence shell.
Will sodium lose 1 electron or gain 7 electrons to obtain a full valence shell?
To fill its valence shell, it would need to acquire 7 electrons - a difficult task for a metal like sodium that is a known electron-loser. So unlike fluorine and oxygen, sodium acquires a full octet by losing electrons and emptying out its outermost shell.
How do you calculate electron shells?
0:344:41Electron shells Elements 1-18 - YouTubeYouTubeStart of suggested clipEnd of suggested clipYou can use the periodic table for the groups 1 2 13 14 15 16 17 and 18 in order to find the numberMoreYou can use the periodic table for the groups 1 2 13 14 15 16 17 and 18 in order to find the number of valence electrons in the outer shell.
Does sodium have 1 valence electrons?
A: An atom of a group 1 element such as sodium has just one valence electron.
What is the inner shell of sodium?
Sodium has one valence electron. The element has a full innermost electron shell of two electrons and a full shell of eight electrons in the next shell. The third shell, which is the outermost and the valence shell, has only one electron.
How many electron shells or energy levels would an element in Period 2 have?
2 energy levelsNumber of energy levels in each period The atoms in the second period have electrons in 2 energy levels. The atoms in the third period have electrons in 3 energy levels. The atoms in the fourth period have electrons in 4 energy levels.
What is the valence electrons for NA?
In your case, sodium is located in the group 1, which means that it has 1 valence electron. This valence electron is located in sodium's third energy shell.
How many electrons can a shell hold?
Shell number one can only hold 2 electrons, shell two can hold 8, and for the first eighteen elements shell three can hold a maximum of eight electrons. As you learn about elements with more than eighteen electrons you will find that shell three can hold more than eight.
What are the electrons in the shells of an atom?
Take a look at the picture below. Each of those colored balls is an electron. In an atom, the electrons spin around the center, also called the nucleus. The electrons like to be in separate shells/orbitals.
How many electrons can be in a shell?
Each successive shell can only hold a certain number of electrons. The innermost shell is filled first. This shell can contain a maximum of two electrons. The second shell can hold a maximum of eight electrons.
How many electrons can a second shell hold?
The second shell can hold a maximum of eight electrons. When this is filled, electrons go into the third shell, which also holds a maximum of eight electrons. Then the fourth shell begins to fill. Energy shell. Maximum number of electrons.
How many electrons does a calcium atom have?
A calcium atom has 20 electrons. Two are in the first shell, eight in the second shell, eight in the third shell, and two in the fourth shell. previous. 1. 2.
Where are electrons arranged in the periodic table?
The electrons are arranged in shells around the nucleus. The periodic table is a chart of all the elements arranged in increasing atomic number. Part of. Chemistry (Single Science) Atomic structure.
