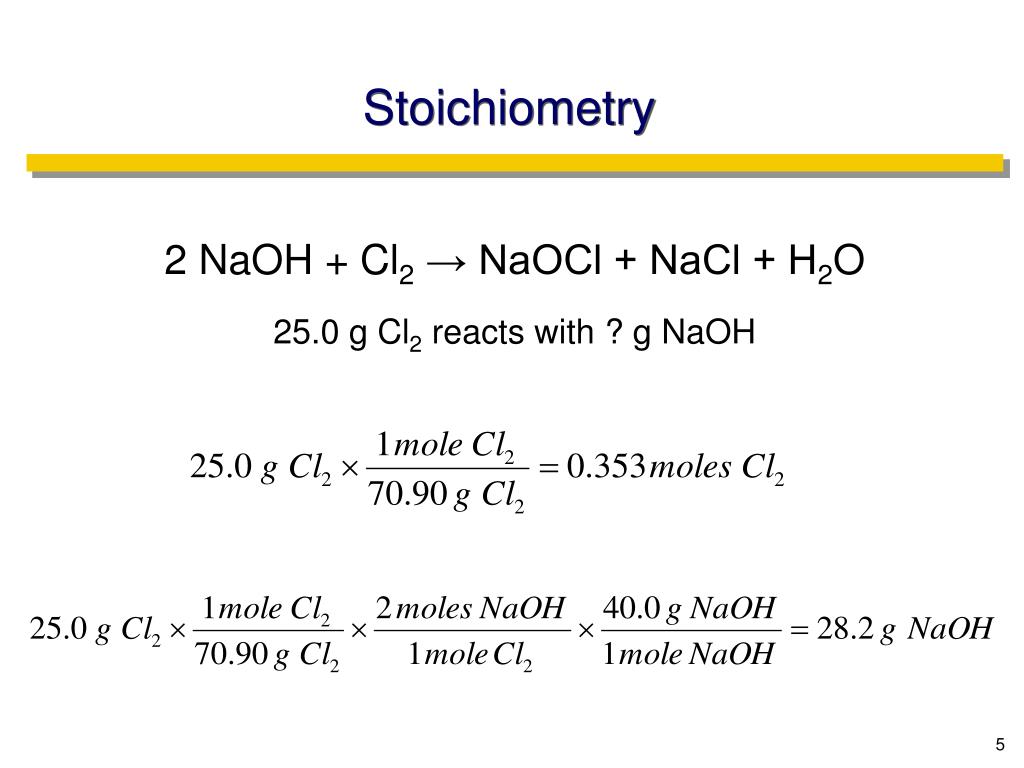
How can the moles of NaOH be calculated?
moles HCL = moles NaOH. We can calculate the number of molesof NaOH at the equivalence point by multiplying its concentration (in terms of molarity or moles/L)by the volume of NaOH added in order to reach the equivalence point: This tells us that there were 0.004398 molesof HCl in our original 0.02500 L solution.
What is the number of moles in NaOH?
The molar mass of NaOH is 40.0 g/mol, so there are 0.75mol⋅40.0g/mol=30g of NaOH, 30. g with the right number of significant figures.
What is the formula for converting grams to moles?
Solution:
- Step One: The problem will tell you how many grams are present. Look for the unit of grams. ...
- Step Two: You need to know the molar mass of the substance. ...
- Step Three: Divide the grams given in the problem by the substance's molar mass: 25.0 g ––––––––– = 0.158 mol 158.034 g/mol The answer of 0.158 mole has been ...
How to calculate molarity NaOH?
… To calculate molarity:
- Find the number of moles of solute dissolved in solution,
- Find the volume of solution in liters, and.
- Divide moles solute by liters solution.

What is the mass of 1 mole of NaOH?
39.997 g/molSodium hydroxide / Molar mass
What is the moles of NaOH?
The moles of NaOH contained in 160g of it is 4 mole.
How many moles of NaOH are in one gram NaOH?
Na mass is 23, O has 16 and H is 1. Add all of these up to get the molar mass of NaOH is 40 g/mol.
What is the gram of NaOH?
Mass of NaOH = 0. 125×40=5g.
How many moles are in 2 grams of NaOH?
Solution : Number of moles `= ("Mass ")/( "Molecular mass ")`
` (2)/(40 )=0.05.
How do you calculate grams to moles?
To correctly estimate the number of moles, n , of a substance of a specific mass, m , (in grams), you need to follow the grams to moles formula: n = m / M , where, M is the molar mass of this material.
How many moles are in 4 grams of NaOH?
This is Expert Verified Answer Hence, 0.100 moles are present in 4 grams of NaOH.
How many moles is 8 grams of NaOH?
0.20The number of moles of the NaOH sample is 0.20.
What is the mass of 2 mole of NaOH?
Solution : 2 mole NaOH
1 mole of NaoH `=40g` of NaOH `=6.023xx10^(22)` molecules
2 mole of NaOH `2xx40g=80g` of NaOH
`=2xx6. 023xx10^(23)` molecules.
How do you make 1M NaOH?
To make 1 M NaOH solution, you have to dissolve 40.00 g of sodium hydroxide pellets in 250 mL distilled water and then make up the solution to 1 liter. Weigh 19.95 gm of NaOH pellets & dissolve them in half liter(500ml) of distilled water water, what you will be having now is 1M NaOH solution.
How do you find the moles of NaOH in a titration?
Step 1: Calculate the amount of sodium hydroxide in molesAmount of solute in mol = concentration in mol/dm 3 × volume in dm 3Amount of sodium hydroxide = 0.100 × 0.0250.= 0.00250 mol.The balanced equation is: NaOH(aq) + HCl(aq) → NaCl(aq) + H 2O(l)So the mole ratio NaOH:HCl is 1:1.More items...
How do I calculate moles?
The unit is denoted by mol.The formula for the number of moles formula is expressed as.Given.Number of moles formula is.Number of moles = Mass of substance / Mass of one mole.Number of moles = 95 / 86.94.
How do you find the moles of NaOH in a titration?
Step 1: Calculate the amount of sodium hydroxide in molesAmount of solute in mol = concentration in mol/dm 3 × volume in dm 3Amount of sodium hydroxide = 0.100 × 0.0250.= 0.00250 mol.The balanced equation is: NaOH(aq) + HCl(aq) → NaCl(aq) + H 2O(l)So the mole ratio NaOH:HCl is 1:1.More items...
How do I calculate moles?
The unit is denoted by mol.The formula for the number of moles formula is expressed as.Given.Number of moles formula is.Number of moles = Mass of substance / Mass of one mole.Number of moles = 95 / 86.94.
How do you make 1M NaOH?
To make 1 M NaOH solution, you have to dissolve 40.00 g of sodium hydroxide pellets in 250 mL distilled water and then make up the solution to 1 liter. Weigh 19.95 gm of NaOH pellets & dissolve them in half liter(500ml) of distilled water water, what you will be having now is 1M NaOH solution.
What is the formula for moles?
Worked Example: moles = mass ÷ molar mass (n=m/M)
How to calculate molar mass?
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
How to find molar mass?
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
How to calculate molecular weight?
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Is molar mass the same as molecular mass?
This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes.
