It is also an SI derived unit
SI derived unit
The International System of Units (SI) specifies a set of seven base units from which all other SI units of measurement are derived. Each of these other units (SI derived units) is either dimensionless or can be expressed as a product of (positive or negative, but usually integral) powers of one or more of the base units.
How many kilojoules are in a mole?
How many Kilogram-mole make 1 Mole? 1 Mole [mol] = 0.001 Kilogram-mole [kg-mol] – Measurement calculator that can be used to convert Mole to Kilogram-mole, among others. Accordingly, Is kJ kg the same as kW?
How to convert Joule to kJ per mole?
How do you convert kilojoules to Grams?
- Kj to gram calorie = 238.90296 gram calorie.
- Kj to gram calorie = 477.80592 gram calorie.
- Kj to gram calorie = 716.70887 gram calorie.
- Kj to gram calorie = 955.61183 gram calorie.
- Kj to gram calorie = 1194.51479 gram calorie.
How many units are in a mole?
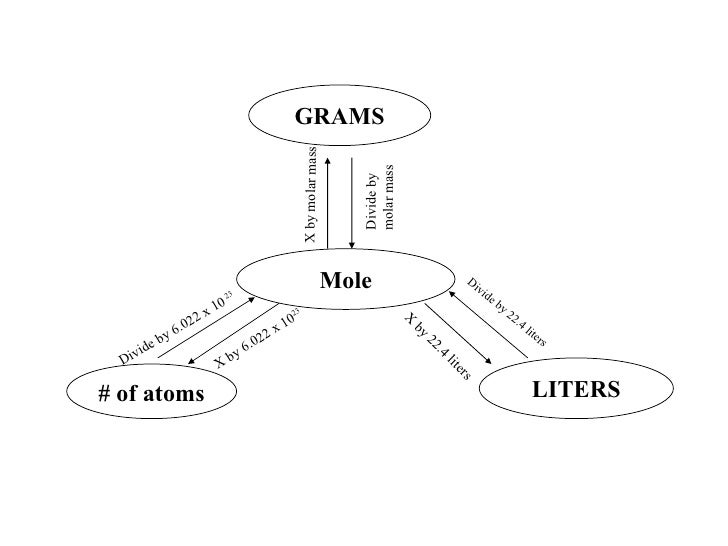
The mole, abbreviated mol, is an SI unit which measures the number of particles in a specific substance. One mole is equal to 6.02214179×1023 atoms, or other elementary units such as molecules. Moreover, what if you had a mole of moles? A Mole of Moles. It's really just a number—like “dozen” or “billion.”
How do you calculate the number of moles from concentration?
How to Find Moles?
- Definition of Number of Moles. It refers to a huge number that we use to measure atoms. ...
- Molarity Calculation. It refers to the number of moles per litre of solution. Moreover, we express it as M. ...
- Solved Question for You. Mole is approximately equal to which of the following? The correct answer is option A.

Are moles a joule of energy?
The joule per mole (symbol: J·mole−1 or J/mol) is an SI derived unit of energy per amount of material. Energy is measured in joules, and the amount of material is measured in moles. In other words, joule per mole is a unit for how much energy is in a certain amount of matter.
How do you convert kJ to kJ mol?
1eV=(1. -22)×(6. =96.49kj/mole.
Is kJ a mol?
Re: Units: kJ vs kJ/mol A chemical reaction is usually kJ because each reaction has its unique number of moles of reactants and products. For example, 2R->3P. However, if one asks what is the change per mole of reactant then divide the number for the reaction (kJ) by 2 mole and the answer is then kJ/mol.
How much is a mole?
One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro's number or Avogadro's constant. The concept of the mole can be used to convert between mass and number of particles.. Created by Sal Khan.
How do you convert kiloJoules per mole to joules per mole?
The formula to convert Kilojoule per Mole to Joule per Mole is 1 Kilojoule per Mole = 1000 Joule per Mole. Kilojoule per Mole is 1000 times Bigger than Joule per Mole.
Is kJ mol the same as kJ?
Usually it seems that delta H for an entire reaction is in kJ, since there are varying numbers of moles depending on which product or reactant it is. If the question asks for kJ per mole of product, then you would have to divide the enthalpy by the specific number of moles and give the answer in kJ/mole.
How do you convert joules to Mols to joules?
Since 1 mole = 6.0221407×1023 particles (atoms, molecules, ions etc.), 1 joule per mole is equal to 1 joule divided by 6.02214076×1023 particles, 1.66054×10−24 joule per particle.
What is the equivalent of 1 joule?
One joule equals the work done (or energy expended) by a force of one newton (N) acting over a distance of one meter (m). One newton equals a force that produces an acceleration of one meter per second (s) per second on a one kilogram (kg) mass. Therefore, one joule equals one newton•meter.
What is J in chemistry?
Illustrated Glossary of Organic Chemistry - J. J (J): (1) The joule. An International System of Units energy unit equal to an applied force of one newton through a distance of one meter, or the energy equivalent to passing an electric current of one ampere through a resistance of one ohm for one second.
What is meant by 1 mole?
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is “mol”.
What is the size of 1 mole?
The number of atoms or other particles in a mole is the same for all substances. The mole is related to the mass of an element in the following way: one mole of carbon-12 atoms has 6.02214076 × 1023 atoms and a mass of 12 grams.
How do you calculate 1 mole?
The unit is denoted by mol.The formula for the number of moles formula is expressed as.Given.Number of moles formula is.Number of moles = Mass of substance / Mass of one mole.Number of moles = 95 / 86.94.
How do you convert kJ to mol?
The prefix "kilo" means 1,000, so one kJ = 1,000 J. As the energies associated with a single molecule or atom are quite small, we often find it easier to discuss the energy found in one mole of the substance, hence "per mole". To get the energy for one molecule, divide kJ/mol by Avogadro's number, 6.022 x 1023.
How do you convert kilojoules to grams and kJ per mole?
As you know, this conversion factor is the molar mass of the given compound. In order to convert kilojoules per gram to kilojoules per mole, you need to multiply by grams per mole.
How do you calculate energy in kJ mol?
The conversion to kJ/mol involves using Avogadro's number and converting J to kJ: E (kJ/mol) = (3.713 × 10−19 J/photon)(6.022 × 1023 photon/mol)(10−3kJ/J) = 224 kJ/mol.
How do you calculate energy per mole?
The equation used to find the energy in a mole of photons is E= hc/lambda where h is Planck's constant, c is the speed of light and is the wavelength of light.
What is the joule?
The joule (symbol J, also called newton meter, watt second, or coulomb volt) is the SI unit of energy and work. The unit is pronounced to rhyme with "tool", and is named in honor of the physicist James Prescott Joule (1818-1889).
What is the symbol for mol?
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is "mol. ".