Full Answer
How many moles are too many?
How many moles are too many? Having more than 11 moles on one arm indicates a higher-than-average risk of skin cancer or melanoma, research suggests. Counting moles on the right arm was found to be a good indicator of total moles on the body. More than 100 indicates five times the normal risk.
How many neutrons are there in mole of a substance?
The result you get is the answer for 1 atom/compound, to find it for a mole of a substance, you multiply the answer with Avogadro’s constant (6.02214086 × 10^23) For example, if you have 1 mole of carbon: The mass number is 12 and the atomic number is 6 so total neutron number is (12–6) × 6.02214086 × 10^23 which is 3.613284516 Continue Reading
How do you calculate the number of photons?
How do you calculate the number of photons emitted per second? According to the equation E=n⋅h⋅ν (energy = number of photons times Planck’s constant times the frequency), if you divide the energy by Planck’s constant, you should get photons per second.
How many joules in one mole?
Since 1 mole = 6.02214179×1023 particles (atoms, molecules, ions etc.), 1 Joule per mole is equal to 1 Joule divided by 6.02214179×1023 particles, or (6.022×10^23 particles/mole), 1.66054×10−24 Joule per particle.

How do you find the moles of a photon?
The equation used to find the energy in a mole of photons is E= hc/lambda where h is Planck's constant, c is the speed of light and is the wavelength of light. The value of E will come out in units that are useful for us to work with if we put the constants and wavelength in the proper units.
How many moles is one photon?
Because photons are particles, they can be counted. The number of photons in a mole of photons is equal to the Avogadro constant, N = 6.022×1023 mol−1 N = 6.022 × 10 23 m o l − 1 . The energy per mole of photons EN of wavelength λ is then EN = N × h × c / λ E N = N × h × c / λ .
What is the value of 1 photon?
The energy of a single photon is: hν or = (h/2π)ω where h is Planck's constant: 6.626 x 10-34 Joule-sec. One photon of visible light contains about 10-19 Joules (not much!)
Can photons be measured in moles?
Light can also be thought of as a stream of particles, photons, or quanta. In this case, units can be expressed in moles per square meter per second (mol m–2 s–1), where "moles" refers to the number of photons (1 mol of light = 6.02 × 1023 photons, Avogadro's number). This measure is called photon irradiance.
Do photons have mass?
A photon is a massless 'particle," meaning it has no rest mass.
How do you find the energy of 1 mol of a photon?
As we know, 1 mole = 6.022 × 1023 atoms.Now the energy of a single photon, E=hν = 6.626×10−34Js ×5×1014s−1.Energy of one mole of photon is E×A. = (3.313 × 10−19 J)(6.022 × 1023 mol−1) = 199.51 kJ mol−1. Hence, option (B) is correct.
How big is a photon?
A photon is in shape like a thin stick if its energy is lower than the rest energy of an electron and like a plate if its radius is smaller than the classical radius of an electron. For a photon of hν=13.6 eV, the photon radius is 34.9 pm and is less than the Bohr radius.
What does a single photon look like?
A photon just looks like a blink of light from a small point. So, when you see a photon (if your eyes are sensitive enough), you see a blip of light. The "size" of a photon is much weirder since photons aren't "particles" in the traditional macroscopic sense of the word.
Is a photon an atom?
Photons and electrons are two of the basic quantum-mechanical particles but they have completely different properties. Photon is a type of elementary particle which acts as a carrier of energy, but the electron is a subatomic particle which occurs in all the atoms.
What is a mole in light?
The cumulative amount of light in a square meter over 24 hours, measured in moles, (or 1,000,000 µmol). This is the same as an instantaneous measurement but totalled for a day's worth of photons, analogous to a 'rain gauge' for light.
How many photons are in a Joule?
It is also useful to calculate the number of photons in a Joule of energy. This is just the inverse of the energy per photon, and gives 3.2x1018 photons per Joule.
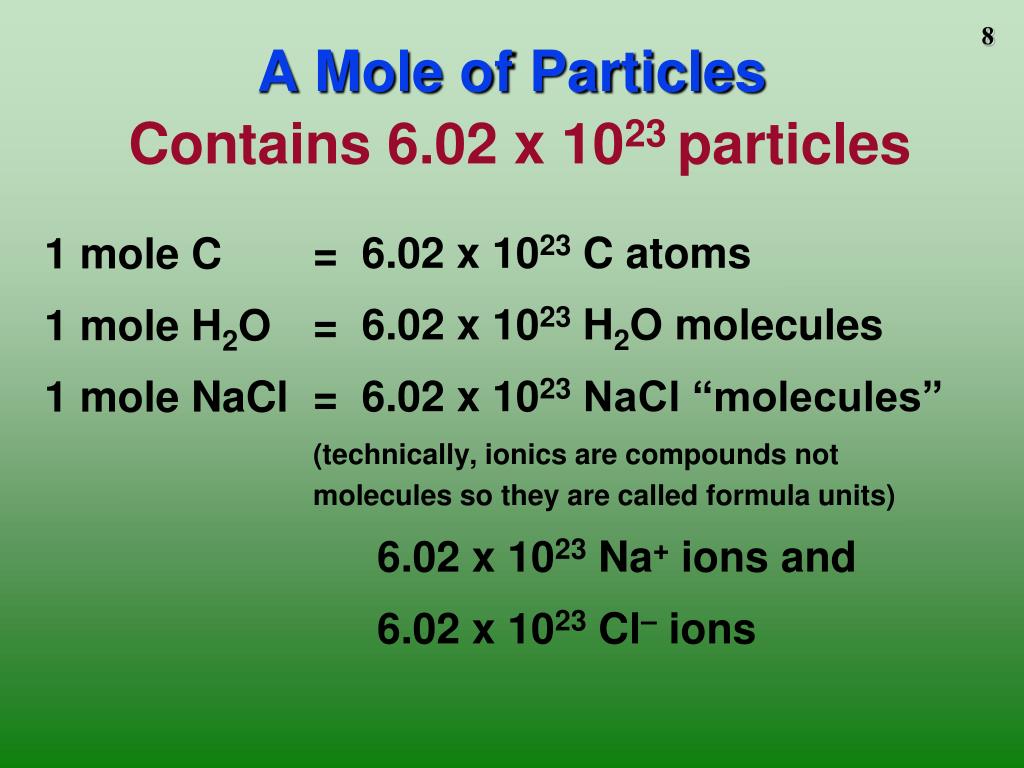
What is 1 mole of a substance?
A mole is the amount (10) of material containing 6.02214 × 1023 particles. ... Notice that the definition of the mole is an amount of substance. We will often refer to the number of moles of substance as the amount of the substance.
How do you calculate the number of photons?
By dividing the total energy of a pulse by the energy of one photon within the pulse, we find the number of photons. The real question then is how to find the pulse's energy and the photon's energy.
How many photons are in a Joule?
It is also useful to calculate the number of photons in a Joule of energy. This is just the inverse of the energy per photon, and gives 3.2x1018 photons per Joule.
What is the energy of a 250 nm photon?
Therefore, 7.95×10−19 J 7.95 × 10 − 19 J is the energy of a photon of ultraviolet radiation with wavelength 250 nm.
How do you calculate the energy of a photon?
The energy of a photon can be calculated in two ways: If the photon's frequency is known, we can use the formula E = h f . Max Planck proposed this equation, which is why it is known as Planck's equation. If the photon's wavelength is known, the photon's energy can be calculated using the formula E = h c λ .
How to calculate the number of photons?
You calculate the energy of a photon, and then you use the total energy to calculate the number of photons.
How many mW does a laser pointer produce?
A common laser pointer produces 1.0 mW at a wavelength of 670 nm. Calculate the number of photons produced per millisecond.
