How many neucleons does tritium have?
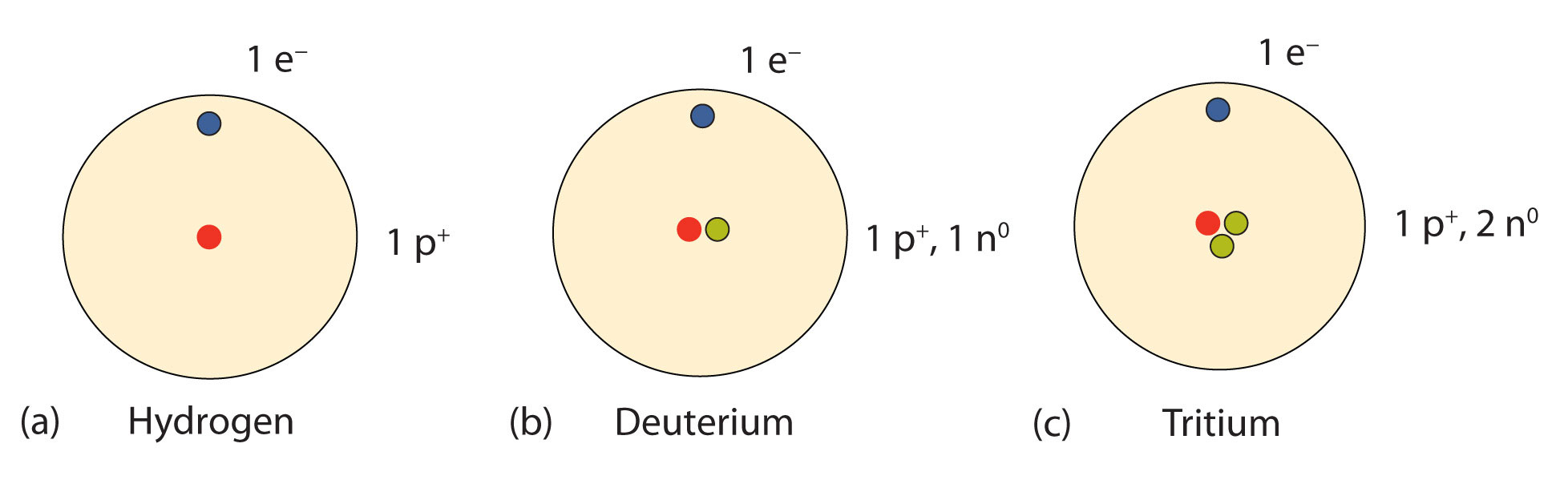
Tritium is a hydrogen isotope consisting of one proton, two neutrons and one electron. It is radioactive, with a half-life of 12.32 years. Click to see full answer.
How many nucleons does tritium have?
The nucleus of tritium (t, sometimes called a triton) contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (protium) contains just one proton, and that of hydrogen-2 (deuterium) contains one proton and one neutron. Naturally occurring tritium is extremely rare on Earth.
What element has 86 electrons 125 neutrons?
What element has, 86 electrons, 125 neutrons, and 82 protons? +2 Answers (1) Cordell Boyle1 September, 05:59 0 The element that contains 86 electrons, 125 neutrons, and 82 protons is lead. Comment Complaint Link Know the Answer? Answer New Questions in Biology Which are ways that humans can lower biodiversity as they use the environment?
What are the number of neutrons in each of elements?
Number of neutrons Solution
- Convert Input (s) to Base Unit
- Evaluate Formula
- Convert Result to Output's Unit
See more

Does tritium have 2 neutrons?
Tritium (abbreviated as 3H) is a hydrogen atom that has two neutrons in the nucleus and one proton. Tritium is produced naturally in the upper atmosphere when cosmic rays strike nitrogen molecules in the air. Tritium is also produced during nuclear weapons explosions, and as a byproduct in nuclear reactors.
How many protons neutrons and electrons does tritium have?
Tritium is an isotope of hydrogen that is composed of one proton, two neutrons, and one electron.
What is the number of protons in tritium?
one protontritium, (T, or 3H), the isotope of hydrogen with atomic weight of approximately 3. Its nucleus, consisting of one proton and two neutrons, has triple the mass of the nucleus of ordinary hydrogen.
How many tritium does deuterium have?
one tritiumDeuterium–tritium fusion (sometimes abbreviated D+T) is a type of nuclear fusion in which one deuterium nucleus fuses with one tritium nucleus, giving one helium nucleus, one free neutron, and 17.6 MeV of energy.
How many protons are in a neutron?
0 1For example, silicon has 14 protons and 14 neutrons. Its atomic number is 14 and its atomic mass is 28. The most common isotope of uranium has 92 protons and 146 neutrons....2.1 Electrons, Protons, Neutrons, and Atoms.Elementary ParticleChargeMassNeutron01Electron−1~01 more row
Why tritium is an isotope?
It is used in a medical and scientific setting as a radioactive tracer. Tritium is also used as a nuclear fusion fuel, along with more abundant deuterium, in tokamak reactors and in hydrogen bombs....Tritium.GeneralBeta emission0.018590Isotopes of hydrogen Complete table of nuclides15 more rows
Is tritium legal in the US?
Although it is illegal for Amazon to support sales of tritium on their site, it is somewhat similar to a game of whack-a-mole. If Amazon finds the listing and shuts it down, another will pop up until they are found again.
Is tritium an element?
Tritium is also known as hydrogen-3 and has an element symbol T or 3H. The nucleus of a tritium atom is called a triton and consists of three particles: one proton and two neutrons. The word tritium comes from Greek the word "tritos", which means "third".
Is tritium Alpha or beta?
beta radiationLike ordinary water, water containing tritium, or tritiated water, is colorless and odorless. Of the three primary types of radiation, alpha, beta and gamma, tritium emits only beta radiation.
Can you drink heavy water?
Made by swapping water's hydrogen atoms with their heavier relative, deuterium, heavy water looks and tastes like regular water and in small doses (no more than five tablespoons for humans) is safe to drink.
Can you make water with tritium?
Like normal hydrogen, tritium can bond with oxygen to form water. When this happens, the resulting "tritiated" water is radioactive. Tritiated water (not to be confused with heavy water) is chemically identical to normal water and the tritium cannot be filtered out of the water.
Is tritium heavy water?
Unsourced material may be challenged and removed. Tritiated water is a radioactive form of water in which the usual protium atoms are replaced with tritium. In its pure form it may be called tritium oxide (T2O or 3H2O) or super-heavy water.
Which element has an isotope with 26 electrons 29 neutrons and 26 protons?
26.
How do you figure out protons neutrons and electrons?
The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus. The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z).
How do you find the number of protons electrons and neutrons?
To calculate the numbers of subatomic particles in an atom, use its atomic number and mass number:number of protons = atomic number.number of electrons = atomic number.number of neutrons = mass number - atomic number.
How many protons does H have?
1Hydrogen / Atomic number
What are the three isotopes of hydrogen?
Three Hydrogen Isotopes: Protium, Deuterium, Tritium. Elements are the building blocks of a chemist’s world. The first and simplest element is hydrogen, H. It is a gas at room temperature. A molecule of hydrogen gas consists of two joined atoms.
What is the most important isotope?
As can be seen from the numbers, the most important isotope is protium or H-1. However, there are special applications for deuterium.
How many atoms are in a hydrogen molecule?
A molecule of hydrogen gas consists of two joined atoms. However, we will discuss the lone atoms, which exist in three varieties: protium, deuterium, and tritium. All hydrogen atoms have an atomic number of 1. This means the central core or nucleus of any of the three varieties of hydrogen contains just 1 proton.
Is tritium a waste?
Tritium is considered a waste product in nuclear reactions. It presents health risks if it reaches an underground water supply. For instance, in the US there was an important tritium leak. See this document from the Brookhaven National Laboratory (BNL). Sometimes, however, tritium is deliberately employed in the armaments business in fission bombs and in the fission component of fusion bombs.
How many protons does a tc have?
Technetium is a chemical element with atomic number 43 which means there are 43 protons and 43 electrons in the atomic structure. The chemical symbol for Technetium is Tc.
What is the mass number of Technetium?
Mass numbers of typical isotopes of Technetium are 97-99.
How many electrons does neon have?
Neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure. The chemical symbol for Neon is Ne.
How many protons and electrons are in hydrogen?
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H.
How many protons does beryllium have?
Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structure. The chemical symbol for Beryllium is Be.
How are atomic nuclei determined?
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). It must be noted, especially nuclear cross-sections may vary by many orders from nuclide with the neutron number N to nuclide with the neutron number N+1. For example, actinides with odd neutron number are usually fissile (fissionable with slow neutrons) while actinides with even neutron number are usually not fissile (but are fissionable with fast neutrons). Heavy nuclei with an even number of protons and an even number of neutrons are (due to Pauli exclusion principle) very stable thanks to the occurrence of ‘paired spin’. On the other hand, nuclei with an odd number of protons and neutrons are mostly unstable.
How do neutrons stabilize the nucleus?
Neutrons stabilize the nucleus, because they attract each other and protons , which helps offset the electrical repulsion between protons. As a result, as the number of protons increases, an increasing ratio of neutrons to protons is needed to form a stable nucleus. If there are too many or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay . Unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay , beta decay , gamma decay or electron capture. Many other rare types of decay, such as spontaneous fission or neutron emission are known.