
How many orbitals does the N-4 level contain?
How many orbitals does the N 4 level contain? l=3 for f subshell. Number of orbitals is = 2l+1=7. It can accomodate a total of 14 electron. Hence for a shell of principal quantum number n=4 there are 16 orbitals ,4 subshells, 32 electrons (maximum) and 14 electrons with l=3. Click to see full answer.
How many subshells are in N=4?
The third shell (n=3) contains 3 subshells (3s, 3p, and 3d), and the fourth shell (n=4) contains 4 subshells (4s, 4p, 4d, and 4f). All d-type subshells have 5 orbitals, regardless of which shell they're in. s-type subshells contain 1 orbital each, p-type subshells contain 3 orbitals each, and f-type subshells contain 7 orbitals each.
How many orbitals have the quantum numbers n?
Hence for a shell of principal quantum number n=4 there are 16 orbitals ,4 subshells, 32 electrons (maximum) and 14 electrons with l=3. First Quantum Number: Orbital and Electron Calculations For n = 2, there are 22 or four orbitals. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 52 = 25 orbitals, and so on.
Are there only 4 types of orbitals?
There are four type of orbitals namely s,p,d,f. The s orbital is the smallest and can accommodate 2 electrons. The p, d, f orbitals can accommodate 6, 10, 14 electrons respectively. The f orbital is the largest. The shape of the s orbital is spherical.
What is the principal quantum number of subshells?
What does l mean in p subshell?
Why are orbitals possible for subshell 7?
How many electrons can one orbital hold?
What is the subshell of n=2?
How many orbitals are there in a shell of n=4?
How many orbitals are there in energy level 4?
See 4 more
About this website
Which types of orbitals exist in the n 4 shell?
Within each shell of an atom there are some combinations of orbitals. In the n=1 shell you only find s orbitals, in the n=2 shell, you have s and p orbitals, in the n=3 shell, you have s, p and d orbitals and in the n=4 up shells you find all four types of orbitals.
What orbitals does energy level 4 have?
The fourth energy level of the periodic table includes the 4s 3d and 4p orbitals. The 4p orbital holds 6 electrons. There is a 4d orbital with 10 electrons which coincides with the 5th energy level of the periodic table.
What is the energy of energy level 4?
-3.4 eVElectrons in a hydrogen atom must be in one of the allowed energy levels. If an electron is in the first energy level, it must have exactly -13.6 eV of energy. If it is in the second energy level, it must have -3.4 eV of energy....Exercise 3.Energy LevelEnergy1-54.4 eV2-13.6 eV3-6.04 eV4-3.4 eV1 more row
How much can the 4th energy level hold?
32 electronsThus, the fourth level can hold up to 32 electrons: 2 in the s orbital, 6 in the three p orbitals, 10 in the five d orbitals, and 14 in the seven f orbitals.
Which element will have 4 energy levels?
Hence, potassium has four energy levels.
What element has an energy level of 4?
Since potassium, calcium, and bromine are in the 4th row or period, their outermost electrons are in the fourth energy level.
What is an element with 4 energy levels?
ElementElement NumberNumber of Electrons in each LevelBeryllium42Boron53Carbon64Nitrogen7551 more rows
How many orbitals does the n=4 shell have ? - Brainly.com
The fourth shell has 4 subshell: the "s" subshell, which has 1 orbital with 2 electrons, the "p" subshell, which has 3 orbitals with 6 electrons, the "d" subshell, which has 5 orbitals with 10 electrons, and the "f" subshell, which has 7 orbitals with 14 electrons, for a total of 16 orbitals and
Solved 2. How many orbitals are present in the n = 4 shell | Chegg.com
Answer to Solved 2. How many orbitals are present in the n = 4 shell
How many orbitals are present in the 4th shell? - Quora
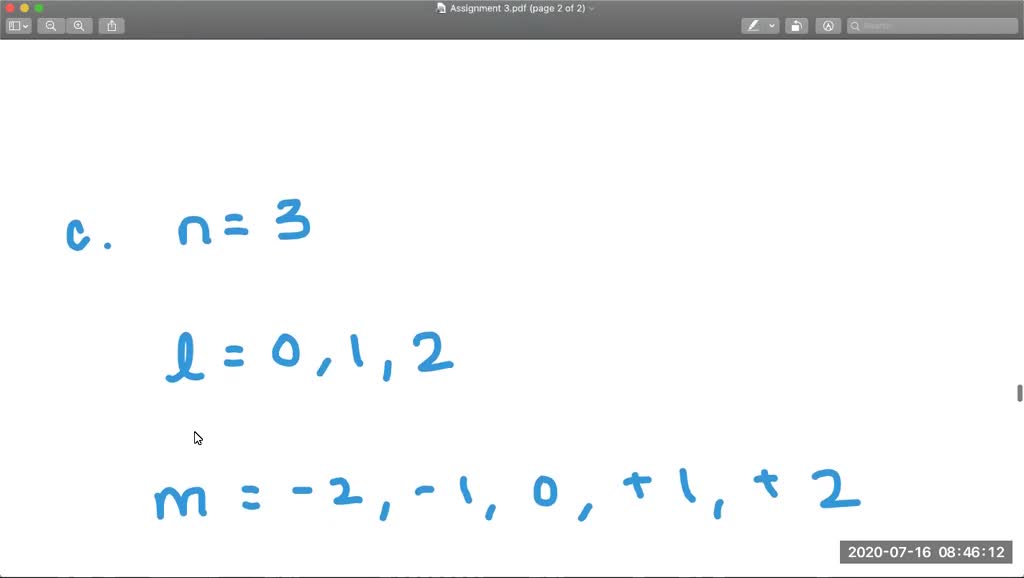
Answer (1 of 2): For 4th shell n=4, ‘l’ can take values n-1 i.e 0,1,2,3 0-s; 1- p; 2-d; 3-f. S has 1 orbital. P has 3. D has 5. F has 7. Total orbitals are 16. Also, n=4. Total no. of orbitals can be calculated as n² =4²=16
How many total orbitals in shell n=4? What is the relationship between ...
n^2 orbitals in each energy level, and n subshells in each energy level. I assume you kind of recognize quantum numbers... n is the principal quantum number, the ...
How many electrons are in n = 2? What about n = 4, l = 3 ... - Socratic
Here's what I got. Since the question is a bit ambiguous, I will assume that you're dealing with three distinct sets of quantum numbers. In addition to this, I will also assume that you're fairly familiar with quantum numbers, so I won't go into too much details about what each represents. 1^"st" set -> n=2 The principal quantum number, n, tells you the energy level on which an electron resides.
What is the principal quantum number of subshells?
For principal quantum number ‘n' the Azimuthal quantum number 'l' which represents subshells is given by n-1. And no. of Subshells possible for that ‘n' is given by 0 to n-1.
What does l mean in p subshell?
For p subshell the l is 1…it means the m lies from -1 to +1. Values for m are -1 , 0, 1. It means three orbitals are possible. px, py and pz.
Why are orbitals possible for subshell 7?
For f subshell 7 orbitals are possible because the m value varies from -3 to +3.
How many electrons can one orbital hold?
Now it has been states that each orbital can hold 2 electrons ( one spin up and one spin down). Thus, 1s ; 2s; 3s; 4s and so one will hold two electrons because they have one orbital.
What is the subshell of n=2?
Similarly for n=2, l is 1. Subshells possible are 0 and 1. Therefore for n=2 these are 2s and 2p. As ' l is 1 for p subshell.
How many orbitals are there in a shell of n=4?
Hence the number of orbitals in n=4 shell is 16.
How many orbitals are there in energy level 4?
So, in energy level 4 there are a total of 16 (1+3+5+7=16) orbitals. It is obvious that the number of orbitals will equal n 2 = 4 2 = 16. So, if we are in energy level 10 there would be total of 10 2 or 100 orbitals. Since each orbital can contain at most 2 electrons, these orbitals could be filled with as many as 200 electrons.
What is the angular momentum quantum number?
l is the angular momentum quantum number, corresponding to the shape of the orbitals of that kind. l = 0,1,2,3,...,n −1. That is, lmax = n − 1.
How many subshells are there in ML?
and each ml value corresponds to one orbital. We have 4 − subshells in this case; s,p,d,f ↔ 0,1,2,3 for the value of l.
How many orbitals does each ML have?
and each ml value corresponds to one orbital. We have 1 − subshell in this case; s ↔ 0 for the value of l.
How many types of orbitals are there in an atom?
There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Within each shell of an atom there are some combinations of orbitals. In the n=1 shell you only find s orbitals, in the n=2 shell, you have s and p orbitals, in the n=3 shell, you have s, p and d orbitals and in the n=4 up shells you find all four types of orbitals. It is important to note here that these orbitals, shells etc. are all part of an empirical theory designed to explain what we observe with respect to molecular structure and bonding. As with any theory, these explanations will only stand as truth until someone (you maybe?) comes up with a better explanation or description.
What is the most likely location of an electron around an atom?
1) An orbital is a three dimensional description of the most likely location of an electron around an atom. Below is a diagram that shows the probability of finding an electron around the nucleus of a hydrogen atom. Notice that the 1s orbital has the highest probability.
What is the principal quantum number of subshells?
For principal quantum number ‘n' the Azimuthal quantum number 'l' which represents subshells is given by n-1. And no. of Subshells possible for that ‘n' is given by 0 to n-1.
What does l mean in p subshell?
For p subshell the l is 1…it means the m lies from -1 to +1. Values for m are -1 , 0, 1. It means three orbitals are possible. px, py and pz.
Why are orbitals possible for subshell 7?
For f subshell 7 orbitals are possible because the m value varies from -3 to +3.
How many electrons can one orbital hold?
Now it has been states that each orbital can hold 2 electrons ( one spin up and one spin down). Thus, 1s ; 2s; 3s; 4s and so one will hold two electrons because they have one orbital.
What is the subshell of n=2?
Similarly for n=2, l is 1. Subshells possible are 0 and 1. Therefore for n=2 these are 2s and 2p. As ' l is 1 for p subshell.
How many orbitals are there in a shell of n=4?
Hence the number of orbitals in n=4 shell is 16.
How many orbitals are there in energy level 4?
So, in energy level 4 there are a total of 16 (1+3+5+7=16) orbitals. It is obvious that the number of orbitals will equal n 2 = 4 2 = 16. So, if we are in energy level 10 there would be total of 10 2 or 100 orbitals. Since each orbital can contain at most 2 electrons, these orbitals could be filled with as many as 200 electrons.
