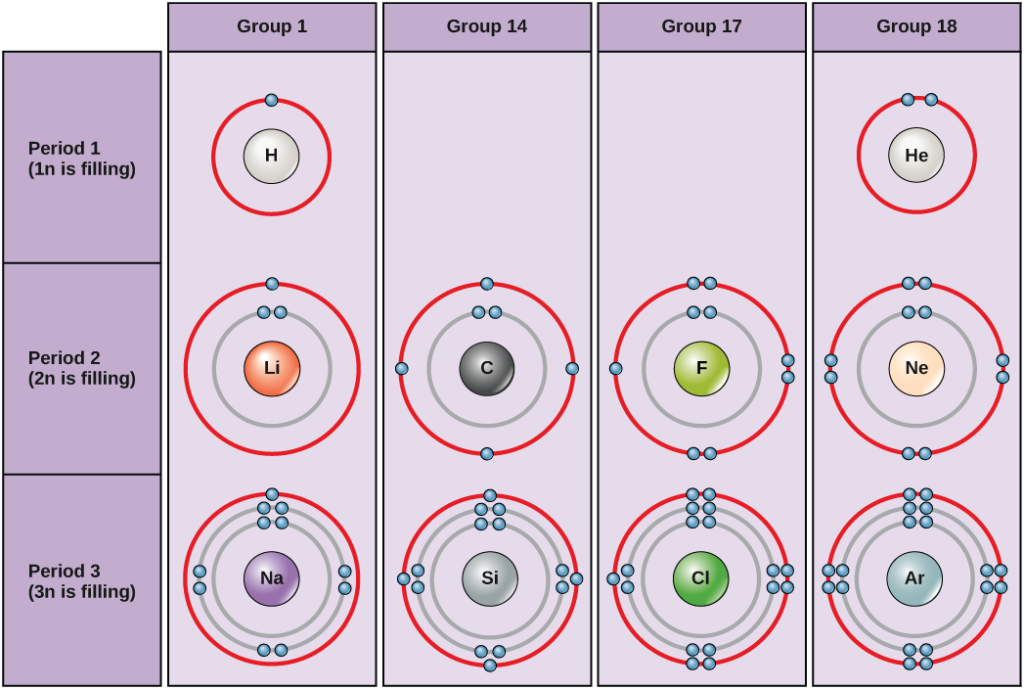
What are the unpaired numbers of electrons in carbon?
- We see that argon is a noble gas.
- Electron configuration: [Ne] 3 (s (2)) 3 (p (6)).
- The p orbital can hold a maximum of 6 electrons.
- In argon we see that the orbitals are completely filled.
- This implies that it does not have any unpaired electrons as all noble gases.
How many protons does carbon have?
Since carbon has an atomic number of 6, it means that it also has 6 protons. The actual definition of carbon is that it’s a chemical element found in the periodic table. It is one of the most abundant elements in the universe. It circulates through the land, atmosphere, and even the ocean, thereby creating what is known as the Carbon Cycle.
How many valence shells does carbon have?
How many valence electrons does each carbon atom have? Two inner shells (core) electrons in the 1s orbital and four valences (outermost shell) electrons in the 2s and 2p orbitals make up atomic carbon’s six electrons. Carbon has four valence electrons to achieve a whole outer energy level by forming four covalent bonds.
How do you write the orbital diagram for carbon?
Carbon is the sixth element with a total of 6 electrons. In writing the electron configuration for carbon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for C goes in the 2s orbital. The remaining two electrons will go in the 2p orbital. Therefore the C electron configuration will be ...

Does carbon have 4 outer electrons?
Carbon has four valence electrons, so it can achieve a full outer energy level by forming four covalent bonds. When it bonds only with hydrogen, it forms compounds called hydrocarbons.
Does carbon have 5 electrons in its outer shell?
Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons, while each hydrogen shares 2. Thus every atom in this stable molecule fulfills the octet rule.
Does carbon have 4 or 6 valence electrons?
1 Answer. Carbon atoms have 4 valence electrons each.
How many electrons does carbon Seek to its outer shell?
To achieve stability, carbon must find four more electrons to fill its outer shell, giving a total of eight and satisfying the octet rule. Carbon atoms may thus form bonds to as many as four other atoms.
How many electron shells does carbon have?
A carbon atom has six electrons. It has two in the first shell and four in the second shell....Electron shells.Energy shellMaximum number of electronsThird82 more rows
How many electrons are in each shell?
The first shell (closest to the nucleus) can hold two electrons. The second shell can hold 8 electrons. The third shell can hold 32 electrons. Within the shells, electrons are further grouped into subshells of four different types, identified as s, p, d, and f in order of increasing energy.
Does carbon have 4 or 8 valence electrons?
The electronic configuration of carbon is 2,4. So, it forms mainly covalent compound as it can neither lose nor gain four-electron to complete its octet. The example of such compound is Methane (CH4).
Does carbon have 2 or 4 valence electrons?
0:121:09Valence Electrons in Carbon (C) - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe can see that there are 4 valence electrons for carbon in the second energy level that's the twoMoreWe can see that there are 4 valence electrons for carbon in the second energy level that's the two plus the two. And that's it that's the number of valence electrons for carbon.
Does carbon have 8 valence electrons?
When bonded with a full octet (such as in methane) carbon has eight valence electrons (two per covalent bond). Each hydrogen atom has two valence electrons.
How many electrons are in the outer shell?
Similarly, the atoms of all group 7 elements have similar chemical properties and reactions to each other, because they all have seven electrons in their outer shell.
What is outermost shell?
The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bond with other atoms. This outermost shell is known as the valence shell & the electrons found in it are called valence electrons.
How many free electrons does carbon have?
It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.
Electron configuration of carbon (C) through orbit
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit (shell). These orbits are expressed by n. [n = 1,2,3,4 .
Electron configuration of carbon (C) atom through orbital
Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by ‘l’. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, f.
How to write the orbital diagram for carbon (C)?
To create an orbital diagram of an atom, you first need to know Hund’s principle and Pauli’s exclusion principle. Hund’s principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way.
Carbon (C) excited state electron configuration
Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of carbon is 1s 2 2s 2 2p 2. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Each sub-orbital can have a maximum of two electrons.
Determination of group and period
The last orbit of an element is the period of that element. The electron configuration of the carbon atom shows that the last orbit of the carbon atom is 2. So, the period of carbon is 2. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element.
Determining the block of carbon by electron configuration
The elements in the periodic table are divided into four blocks based on the electron configuration of the element. The block of elements is determined based on the electron configuration of the element. If the last electron enters the p-orbital after the electron configuration of the element, then that element is called the p-block element.
Activation sequence of carbon atom
The elements of group-14 are relatively less active. However, the activity of the element increases as it moves from the top to the bottom of the group. Carbon is the first element of group-14. Carbon is the number one element in group-14. For this, the activation of carbon atoms is very low.

How Many Electrons Does Carbon have? The Configuration
- Carbon has the atomic numberZ of 6. Therefore, no protons equal the number of electrons equals the atomic number of an atom when it is neutral. Carbon has six electrons with the configuration 1s2 2s2 2p2, where the 2s2 and 2p2 can engage in hybridization and give four unpaired electrons. As a result, carbon has two valences: 2,4.
What Is Carbon?
- Carbon is the 15th most plentiful element in the Earth’s crust and, by mass, the fourth most common element in the universe, followed by hydrogen, helium with oxygen. Carbon’s mass, the unique diversity of organic combinations, and rare ability to form polymers at common Earth temperatures allow it to serve as a common ingredient of all known life. After oxygen, it is the se…
Carbon and Its History
- Carbon was first discovered in prehistory and was known to be the oldest human civilization in the forms of soot and charcoal. Diamondswere undoubtedly discovered in China as early as 2500 BCE, while carbon in the form of charcoal was created during the time of the Romans using the same chemistry as it is today by heating wood in a pyramid coated with clay to exclude air. Ren…
Conclusion
- Carbon is the most abundant element on Earth, accounting for more than half of the planet’s crust. It is a strong element with various applications, ranging from being in our bodies to be a crucial ingredient in many industrial processes. We believe that you got a clear idea about how many electrons does carbon have and the history behind carbon.