How many atoms of oxygen are there in NO3?
There are nine atoms of oxygen because there are 3 molecules of NO3 (nitrate), and each molecule contains 3 oxygen atoms.
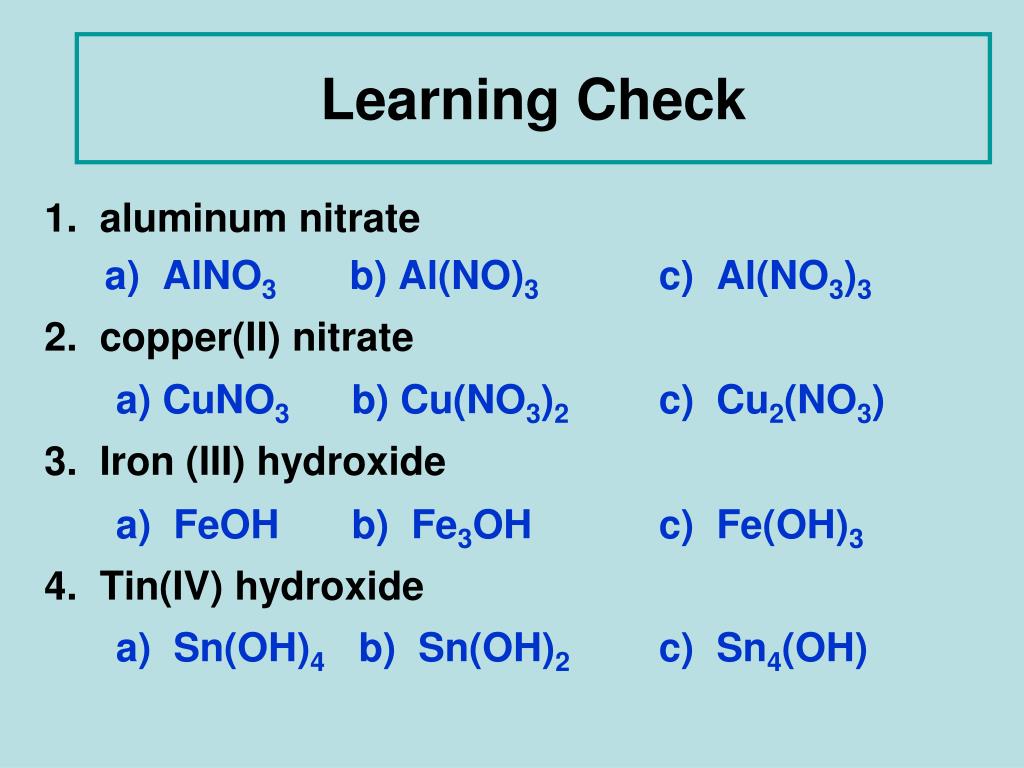
How many atoms are in Al(NO3)3?
- Answers How many atoms in AL (NO3)3? its Al + NO3 + NO3 + NO3. So one Al, three N and nine O. That makes 13 atoms.
How many atoms are there in 3H2O?
first of all 3H2O is not a molecular formula. it represents 3 molecules of H2O which then have 9 atoms How do I calculate the number of atoms of 3Al2 (SO4) 3? So, the total number of atoms in one molecule is 17 atoms. How many atoms and ions are in 3 Al2 (SO4)3? 3 times Al Al SO4. SO4. SO4
How many ions are in 3 molecules of ni3o4?
Ions: there are 2 Al ions and 3 sulphate ions ineach molecule → 5 ions per molecule. So for 3 molecules multiply 5 by 3 Medical Course from GOVT. BOYS MODEL HIGHER SECONDARY SCHOOL KULGAM,KASHMIR,INDIA Author has 299 answers and 2.4M answer views 8 mo How many atoms are in Ni3O4? There are two atoms in Ni3O4 i.e; Nickel and Oxygen.
What to do if material is on fire?
What is a large fire?
How far should you isolate spills in ERG 140?
What are the hazards of combustion products?
How to control a large fire?
What happens if you burn a combustible material?
What is the odour threshold for aluminum nitrate?
See 4 more
About this website

How many moles of oxygen atoms are in Al NO3 3?
So there's three groups of three. So altogether there's nine oxygen's Per one aluminum nitrate. And so for talking on units of moles, one mole of aluminum nitrate will contain nine moles of oxygen, So nine is going to be the answer, Which is # two.
How many atoms are in the formula Al NO3 3?
There is one aluminium atom. 3×(NO3) 3 × ( N O 3 ) , having 3 nitrogen atoms and 9 oxygen atoms. Hence one unit of aluminium nitrate contains total 13 atoms: 1 atom of aluminium.
How many oxygen atoms are there in NO3?
three oxygen atomsNO3 is the nitrate ion. It consists of a nitrogen atom and three oxygen atoms.
How many oxygen atoms are in Al NO3 2?
Looking at the chemical formula of aluminum nitrate, we see that each formula unit of aluminum nitrate contains three nitrate ions. And each nitrate ion contains three oxygen atoms. This means that one formula unit of aluminum nitrate has nine oxygen atoms.
How many atoms do oxygen have?
2 atomsA molecule of oxygen contains 2 atoms. Oxygen is a colourless, odourless, tasteless gas essential to living organisms.
How many atoms of oxygen are there in one formula unit?
two oxygen atomsOne mole of oxygen gas, which has the formula O2, has a mass of 32 g and contains 6.02 X 1023 molecules of oxygen but 12.04 X 1023 (2 X 6.02 X 1023) atoms, because each molecule of oxygen contains two oxygen atoms.
How many moles of oxygen are in NO3?
Examining (NO3)2 only, we see that the subscript 3 indicates 3 mol s of oxygen atoms within each mol of NO−3 (nitrate) polyatomic ions.
How do you find the number of oxygen atoms in a compound?
1:167:19WCLN - Counting Oxygen Atoms - Chemistry - YouTubeYouTubeStart of suggested clipEnd of suggested clipMeans that subscripts inside the bracket must be multiplied by two in order to get the number ofMoreMeans that subscripts inside the bracket must be multiplied by two in order to get the number of atoms of an element. To find the number of oxygen atoms. We go 3 times 2 equals 6.
How many and what type of atoms are present in a nitrate ion NO3 -?
In nitrate ion one nitrogen atom and three oxygen atoms are present.
How many atoms of oxygen are in Al2O3?
Alumina is used in circuit and computer components, as well as micro-abrasives. The α-Al2O3 unit cell has a hexagonal cell structure, with a rhombehedral primitive cell consisting of 10 atoms. Four of these atoms are Aluminum, six are Oxygen.
How many N atoms are in the expression 2al NO3 3?
0:441:27How to Find the Number of Atoms in Al(NO3)3 (Aluminum nitrate) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo a total of 13 atoms in aluminum nitrate.MoreSo a total of 13 atoms in aluminum nitrate.
What is the number of oxygen atoms in Al 2 SO4 3?
12 oxygen atomsSo there are two aluminum atoms, three sulfur atoms, and 12 oxygen atoms in a single unit of the compound Al2(SO4)3.
How many atoms of each element are in 2al NO3 3?
Explanation: In 1 formula unit of Al(NO3)3 , there are (clearly!) 9 atoms of oxygen, 3 nitrogen atoms, and 1 aluminum atom.
How many atoms of each element are present in Al2 co3 3?
So we have to Aluminum atoms and three carbonate molecules. So number of aluminum atoms is two and number of carbonate molecules is three, which means number of carbon atoms Will be three, and number of oxygen atoms will be three and 2.
How many total atoms are in the formula Al co3 3?
0:371:23How to Find the Number of Atoms in Al2(CO3)3 (Aluminum carbonate)YouTubeStart of suggested clipEnd of suggested clipThree times three nine oxygen atoms so in aluminum carbonate we have nine plus three that's 12 plusMoreThree times three nine oxygen atoms so in aluminum carbonate we have nine plus three that's 12 plus two 14 total atoms in al2 co3 sometimes you're asked how many say carbon atoms are in one mole of
What is the formula for Al 3 and NO3?
Aluminium nitratePubChem CID26053Molecular FormulaAl(NO3)3 or AlN3O9SynonymsAluminium nitrate Aluminum trinitrate 13473-90-0 aluminum;trinitrate HUO854648Y More...Molecular Weight213.00Component CompoundsCID 5359268 (Aluminum) CID 944 (Nitric Acid)4 more rows
Molecular weight of Aluminium Nitrate
››Aluminium Nitrate molecular weight. Molar mass of Al(NO3)3 = 212.996238 g/mol Convert grams Aluminium Nitrate to moles or moles Aluminium Nitrate to grams. Molecular weight calculation: 26.981538 + (14.0067 + 15.9994*3)*3
Aluminium nitrate - Wikipedia
Nitric Aluminum salt aluminum nitrate aluminium(III) nitrate
Aluminium Nitrate Formula - Properties, Reactions and Uses - VEDANTU
Aluminium nitrate is a solid white coloured inorganic chemical compound. A nitrate is any polyatomic ion made up of oxygen and nitrogen having a chemical formula NO 3-, which clearly shows that it is an anion with one negative charge. It is well known that aluminium atoms are capable of donating three electrons to form a cation, in order to achieve stable electronic configuration.
How many molecular ions are in SO4?
So 3 molecular ions of SO4 for 2 atoms of Al, means 1.5 Avogadro’s number molecular ions of SO4 for 1 Avogadro’s number atoms of Al that is 9.03 * 10^23 molecular ions of SO4. So 9.03 * 10^23 molecular ions of SO4 will be required.
How many carbonate ions are in Al2?
Finally there are three carbonate ions in Al2 (CO3)3.
How many valence electrons does a carbonate ion have?
In the carbonate ion, there is one carbon atom and three oxygen atoms (ions) as one carbon atom share it's four valence electrons with two O (2-) ions and thus makes charge as zero. Another oxygen ion O (2-) is partially bonded with the CO2 molecule thus making CO3 (2-).
What is the charge of aluminium ion?
The charge of the aluminium ion is 3+ and charge of the CO3 (carbonate) ion is 2- and thus two aluminium ions combine with three carbonate ions.
What is the definition of molality?
Molality is defined as number of moles of solute present in a kg of solvent.
What is the least amount of product in a limiting reactant problem?
In a limiting reactant problem we can use each reactant to compute the amount of product. The least amount of product will be the theoretical yield and indicate the limiting reactant.
How to find the number of moles of oxygen in a compound?
Now that we have the percent composition of Oxygen, we can use this percentage to find the number of moles of oxygen in the compound by multiplying the percentage of oxygen composition and the total number of moles.
What is the formula for calculating molar mass?
If the formula used in calculating molar mass is the molecular formula , the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom ...
How to find the formula weight?
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
What is the formula weight of a compound?
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction.
Is molar mass the same as molecular mass?
This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight ...
What to do if material is on fire?
If material on fire or involved in fire: Flood with water. Cool all affected containers with flooding quantities of water. Apply water from as far a distance as possible.
What is a large fire?
LARGE FIRE: Flood fire area with water from a distance. Do not move cargo or vehicle if cargo has been exposed to heat. Move containers from fire area if you can do it without risk. FIRE INVOLVING TANKS OR CAR/TRAILER LOADS: Fight fire from maximum distance or use unmanned hose holders or monitor nozzles.
How far should you isolate spills in ERG 140?
Excerpt from ERG Guide 140 [Oxidizers]: As an immediate precautionary measure, isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids. LARGE SPILL: Consider initial downwind evacuation for at least 100 meters (330 feet). FIRE: If tank, rail car or tank truck is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2016)
What are the hazards of combustion products?
Special Hazards of Combustion Products: Toxic oxides of nitrogen may form in fire. Behavior in Fire: May increase the intensity of fire when in contact with combustible material (USCG, 1999)
How to control a large fire?
Do not use dry chemicals or foams. CO2 or Halon ® may provide limited control. LARGE FIRE: Flood fire area with water from a distance. Do not move cargo or vehicle if cargo has been exposed to heat. Move containers from fire area if you can do it without risk. FIRE INVOLVING TANKS OR CAR/TRAILER LOADS: Fight fire from maximum distance or use unmanned hose holders or monitor nozzles. Cool containers with flooding quantities of water until well after fire is out. ALWAYS stay away from tanks engulfed in fire. For massive fire, use unmanned hose holders or monitor nozzles; if this is impossible, withdraw from area and let fire burn. (ERG, 2016)
What happens if you burn a combustible material?
If large quantities are involved or the combustible material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Fires that involve this material, produce oxides of nitrogen. Uses include, petroleum refining, dyeing, and leather tanning.
What is the odour threshold for aluminum nitrate?
Odor perception threshold for aluminum nitrate appeared to be the concentration of 0.1 mg/L.