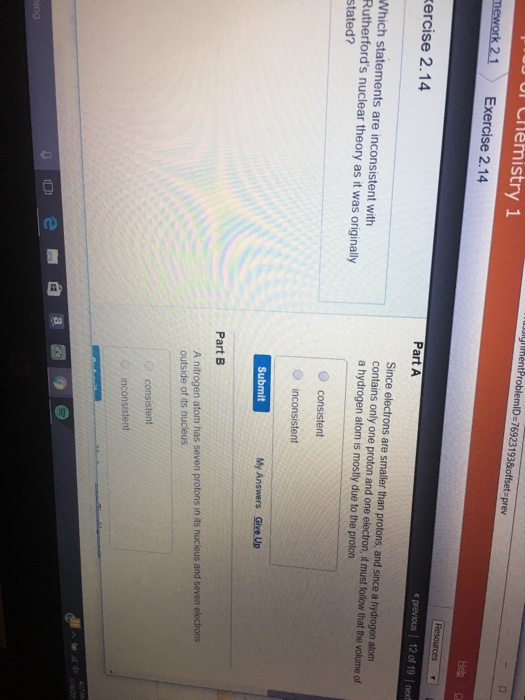
How many protons neutrons and electrons are in a nickel atom?
Nickel-60 is composed of 28 protons, 32 neutrons, and 28 electrons. Nickel-61 is composed of 28 protons, 33 neutrons, and 28 electrons. Nickel-61 is the only stable isotope of nickel with a nuclear spin (I = 3/2), which makes it useful for studies by EPR spectroscopy. Nickel-62 is composed of 28 protons, 34 neutrons, and 28 electrons.
What is the number of electrons in a neutral atom of nickel-64?
Nickel-64 is composed of 28 protons, 36 neutrons, and 28 electrons. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Nickel is 28.
What are the mass numbers of Typical isotopes of nickel?
Mass numbers of typical isotopes of Nickel are 60; 61; 62; 64. Naturally occurring nickel is composed of five stable isotopes; 58Ni , 60Ni , 61Ni , 62Ni and 64Ni, with 58Ni being the most abundant (68.077% natural abundance). Nickel-58 is composed of 28 protons, 30 neutrons, and 28 electrons.
How do you find the number of particles in a nucleus?
To calculate the numbers of subatomic particles in an atom, use its atomic number and mass number: number of protons = atomic number. number of electrons = atomic number. How do you find the number of particles in an element?
How many protons does strontium have?
What are the two main forces in an atom?
Why was the electron discovered first?
Which model of the atom includes a dense central nucleus with protons, positive charge and all the mass?
About this website

How many particles are in the nucleus of an atom?
The nucleus, with its four protons and five neutrons, is surrounded by a cloud of electrons. In 1911, Ernest Rutherford discovered that at the core of every atom is a nucleus. Atomic nuclei consist of electrically positive protons and electrically neutral neutrons.
How many particles are in the nucleus of an atom of copper?
Copper has an atomic number of 29 and a mass number of 63. The atomic number is the number of protons in the nucleus, and the mass number is the number of protons and neutrons in the nucleus. This means that the copper atom has 29 protons and 34 neutrons in its nucleus (29 + 34 = 63).
How do you find the number of particles in a nucleus?
To calculate the numbers of subatomic particles in an atom, use its atomic number and mass number: number of protons = atomic number. number of electrons = atomic number. number of neutrons = mass number - atomic number.
Does nickel have 30 or 31 neutrons?
Nickel is the first element in the tenth column of the periodic table. It is classified as a transition metal. Nickel atoms have 28 electrons and 28 protons with 30 neutrons in the most abundant isotope.
How many particles are there in copper?
1 Answer. There are 29 protons, 35 neutrons, and 29 electrons in a copper atom.
How many particles are in the nucleus of an atom of gold?
1 Answer. A Gold (Au) atom has 79 protons and 79 electrons. A typical gold atom has 118 neutrons, though there are 18 other radioisotopes discovered so far. 79 is its charge (atomic number), which is both its proton number and electron number.
How do you calculate number of particles?
Formula: number of particles = number of moles x 6 x 10 Since 1 mole of substance contains 6 x 1023 particles, 2 moles of substance contains 2 x 6 x 1023 particles. 0.5 moles of substance contains 0.5 x 6 x 1023 particles.
What are the 3 particles in the nucleus of an atom?
There are three subatomic particles: protons, neutrons and electrons. Two of the subatomic particles have electrical charges: protons have a positive charge while electrons have a negative charge. Neutrons, on the other hand, don't have a charge.
How do you calculate particles?
Calculating the number of particlesThe number of particles in a substance can be calculated using:Number of particles = Avogadro constant × the amount of substance in mol.Calculate the number of water molecules in 0.5 mol of water.Number of water molecules = Avogadro constant x amount of substance in mol.
Does nickel have 28 neutrons?
A typical nickel atom has 28 electrons (negative charges) surrounding a nucleus of 28 protons (positively charged nucleons) and 30 neutrons (neutral nucleons).
What element has 31 protons in its nucleus?
Gallium is the chemical element with the atomic number 31 and symbol Ga on the periodic table. It is in the Boron family (group 13) and in period 4.
What has 28 protons and 31 neutrons?
NickelNameNickelNumber of Protons28Number of Neutrons31Number of Electrons28Melting Point1453.0° C9 more rows
What is the nucleus of a copper atom?
All copper atoms have atomic number 29: all their nuclei contain 29 protons. But they also contain uncharged particles called neutrons. In natural copper, the atoms are of two kinds. One has 29 protons and 34 neutrons in the nucleus; the other has 29 protons and 36 neutrons (Figure 4).
How many electrons are in the nucleus of copper?
29 electronsThe copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus.
How many atoms are there in copper?
Concept 2. The relation between molecular (formula) mass and molar mass Page 4 4 • To obtain one mole of copper atoms (6.02 x 1023 atoms), weigh out 63.55 g copper. The molar mass (M) of a substance is the mass of one mole of entities (atoms, molecules, or formula units) of the substance.
How does the nucleus of a copper atom compared to?
How does the nucleus of a copper atom compare to the nucleus of a nickel atom? The nickel's nucleus contains 28 protons and the copper contains 29 protons, since they're one period apart.
How many protons does strontium have?
An atom of strontium and an ion of strontium both have 38 protons in their nucleus and if they have the same mass number both atoms have the same number of neutrons. The strontium with a +2 charge will have 2 fewer electrons around the nucleus (36) than the strontium atom (38).
What are the two main forces in an atom?
There are two main forces in an atom, electrostatic force and nuclear strong force. Describe each one in terms of the particles it affects, and whether it is an attractive force or a repulsive force.
Why was the electron discovered first?
The electron was discovered first because it is on the outside of the atom, not buried in the nucleus and it has a negative charge.
Which model of the atom includes a dense central nucleus with protons, positive charge and all the mass?
The nuclear model of the atom includes a dense central nucleus with protons, positive charge and all the mass. Electrons move around the outside of the nucleus and have negative charge.
How many protons does strontium have?
An atom of strontium and an ion of strontium both have 38 protons in their nucleus and if they have the same mass number both atoms have the same number of neutrons. The strontium with a +2 charge will have 2 fewer electrons around the nucleus (36) than the strontium atom (38).
What are the two main forces in an atom?
There are two main forces in an atom, electrostatic force and nuclear strong force. Describe each one in terms of the particles it affects, and whether it is an attractive force or a repulsive force.
Why was the electron discovered first?
The electron was discovered first because it is on the outside of the atom, not buried in the nucleus and it has a negative charge.
Which model of the atom includes a dense central nucleus with protons, positive charge and all the mass?
The nuclear model of the atom includes a dense central nucleus with protons, positive charge and all the mass. Electrons move around the outside of the nucleus and have negative charge.