Why are there infinitely many photons?
What state of energy is a photon bound in?
Why won't fundamental particles emit photons?
Can an elementary particle absorb a photon?
Can a bound electron absorb a photon?
See 2 more
About this website

How do you calculate the number of absorbed photons?
How can I calculate the total number of photons absorbed by sample (sample with different concentrations)? E photon was calculated by (Power intensity*time of irradiation*wavelength)/(plank constant*light velocity).
How many photons can atoms absorb?
Physicists have long known that a single atom can absorb or emit two photons simultaneously. These two-photon, one-atom processes are widely used for spectroscopy and for the production of entangled photons used in quantum devices.
How many photons do electrons absorb?
one photonEach electron can absorb energy by absorbing one photon when irradiated by electromagnetic energy, but as they adhere to an "all or nothing" code of conduct, all of the energy from that one photon must be absorbed and used to free one electron from atomic binding, or the energy must be re-emitted - the photon must be ...
Are all photons absorbed?
If there is no such dissipative mechanism, providing a store where the excess energy shifted by the photon can be dissipated, then only photons with a narrow band of energies will be absorbed. This is often called a resonant effect, and is much studied in further physics.
Can an electron absorb more than one photon?
"The electrons could not absorb more than one photon to escape from the surface, they could not therefore absorb one quanta and then another to make up the required amount – it was as if they could only embrace one quantum at a time.
Are photons created and destroyed?
Photons are easily created and destroyed. An electron moving in a strong magnetic field will generate photons just from its acceleration. Similarly, when a photon of the right wavelength strikes an atom, it disappears and imparts all its energy to kicking the electron into a new energy level.
Can a proton absorb a photon?
The proton has to be absorbing and emitting photons for it be exerting an electrical force on the electron. Most of those are virtual photons, but clearly the proton is capable of absorbing a photon for some value of "photon" and "absorb".
Do atoms absorb photons?
An atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light.
How many photons are in a atom?
A single atom, by its nature, can only emit one photon at a time. A single photon can be generated at will by applying a laser pulse to a trapped atom. By putting a single atom between two highly reflective mirrors, a so called cavity, all of these photons are sent in the same direction.
Where do all the photons go?
The simplest answer is that when a photon is absorbed by an electron, it is completely destroyed. All its energy is imparted to the electron, which instantly jumps to a new energy level.
Where do photons go when you turn off the light?
The photons – those that were emitted before the lamp was turned off – continue bouncing off objects until they're completely absorbed by stuff inside the room. In a fraction of a millisecond, all the photons are completely absorbed within the room.
How many photons are in the universe?
Now, by using an indirect method, scientists have finally made this measurement. The team found that the amount of starlight, or the number of photons (particles of visible light) that stars have emitted throughout the history of the observable universe is 4×10^84 photons.
Can atoms absorb photons?
An atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light.
How many photons are in a atom?
A single atom, by its nature, can only emit one photon at a time. A single photon can be generated at will by applying a laser pulse to a trapped atom. By putting a single atom between two highly reflective mirrors, a so called cavity, all of these photons are sent in the same direction.
What happens when atoms absorb photons?
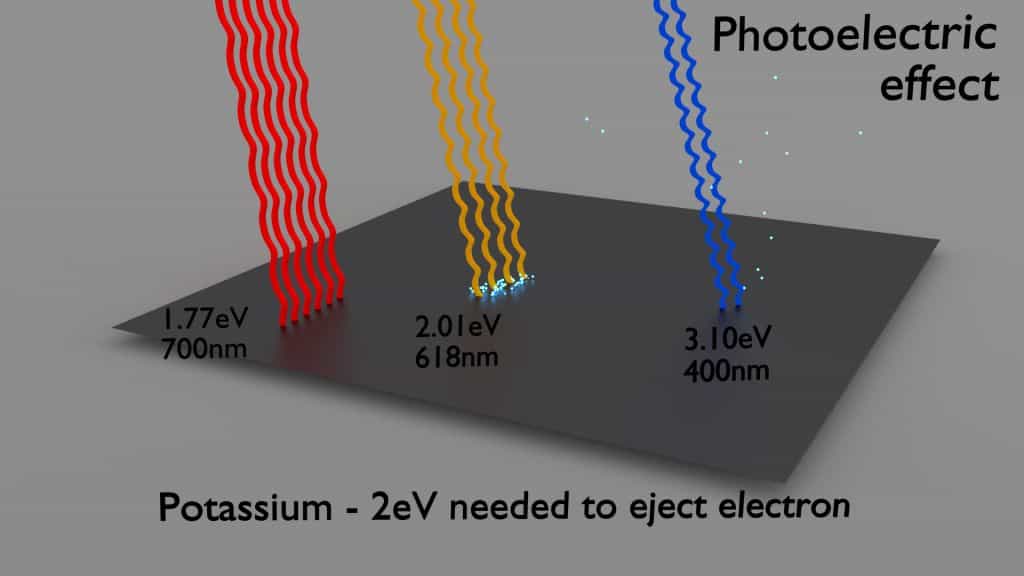
Photon absorption by an atomic electron occurs in the photoelectric effect process, in which the photon loses its entire energy to an atomic electron which is in turn liberated from the atom. This process requires the incident photon to have an energy greater than the binding energy of an orbital electron.
What happens to the atom if the photon is absorbed?
When an electron is hit by a photon of light, it absorbs the quanta of energy the photon was carrying and moves to a higher energy state. One way of thinking about this higher energy state is to imagine that the electron is now moving faster, (it has just been "hit" by a rapidly moving photon).
How many photons can an electron hold at one time? - Quora
Answer (1 of 3): An electron does not hold photons because photons vary in energy by a factor of some 20 orders of magnitude and what is emitted depends on conditions prevailing at that instant. Simple arithmetic shows the equivalent energy content of the rest mass of an electron is roughly 10^7...
How many photons can be emitted by a single electron? - Quora
Answer (1 of 12): As many as you like. If you are concerned that each photon carries away energy and momentum of the electron, think of that some other source of energy initially caused the acceleration of the electron. For example, an electromagnetic field that caused it should be “corrected” w...
Solved How many photons are absorbed by a single atom in - Chegg
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer See Answer See Answer done loading
Solved How many photons are released by a single atom in - Chegg
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer See Answer See Answer done loading
Electrons and Photons - Science in Your Life
This new information explains that electrons are the source of the electromagnetic force and the speed of light that is given to the photon.
Absorption spectrum (emission spectrum lines) (article) | Khan Academy
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
How many photons are in 50 keV?
OK, it seems that 50 keV is the energy of a single photon. You don't have 402 photons totaling 50 keV of energy. You need to find how many Joules are absorbed by the body and then figure out how many 50 keV photons must be absorbed to amount to that much energy.
How much energy does an x-ray have?
The energy of the x-ray is 50 keV, how many photon has been absorbed?
How much msv is in a body part with a mass of 1,2 kg?
The additional information that I forgot to put in is that a body part with the mass of 1,2 kg is being x-rayed and gets an equivalent dose of 0,40 mSv.
How to calculate the number of photons?
You calculate the energy of a photon, and then you use the total energy to calculate the number of photons.
How many mW does a laser pointer produce?
A common laser pointer produces 1.0 mW at a wavelength of 670 nm. Calculate the number of photons produced per millisecond.
Why are there infinitely many photons?
Unboundedly many, because photon number is not conserved. Every time you push an electron with a classical field, you produce infinitely many soft-photons (if the universe is flat at infinity) and conversely, any long range field which pushes the electron has infinitely many soft-photons getting absorbed in a sense, although you can't tell photons apart, so you can't distinguish the ones that were absorbed from the ones that were emitted.
What state of energy is a photon bound in?
Electrons which are bound in atoms or molecules (or even crystals) are in a quantized state of energy. A photon with the appropriate energy can kick up to a higher quantized level the electron and then it will be absorbed/disappear.
Why won't fundamental particles emit photons?
The fundamental particle won't emit photons as it did not absorb one in the first place.
Can an elementary particle absorb a photon?
If you read the links provided you will understand that an elementary particle does not absorba photon, it can interactwith a photon and the result can be variable, but there will always be two particles in and two particles out, because of momentum conservation. The possible results of a photon interacting with an electron are drawn as Feynman diagrams. The same electron in its trajectory can interact with an unlimited number of photons.
Can a bound electron absorb a photon?
A bound electron can absorb a complete photon as we see in photoelectric effect.
