
What are the health hazards of benzene?
What are the stability and reactivity hazards of benzene?
- Chemical Stability: Normally stable.
- Conditions to Avoid: Open flames, sparks, static discharge, heat and other ignition sources.
- Incompatible Materials: Reacts explosively with: halogens (e.g. chlorine). ...
- Hazardous Decomposition Products: None known.
- Possibility of Hazardous Reactions: None known.
What are the harmful effects of benzene?
- Depletion of intracellular glutathione – a critical antioxidant
- Generation of oxygen free radicals
- Induction of apoptosis or cellular death
- DNA damage
- Altered differentiation in progenitor cells
- Depletion of the stem cell pool
What are the risks of benzene?
What are the health risks of benzene?
- Respiratory tract irritation
- Irritation of eyes, skin and mucous membranes
- Dizziness
- Drowsiness
- Abnormal heart rate
- Loss of consciousness
Why is benzene so toxic despite being so stable?
Scientific studies have found that after a long period away from the aircraft, our ability to react quickly is retained better than our ability to act reliably. This means that we get up to speed flying the aircraft quickly, but we are three times more likely to make errors in doing so.
See more
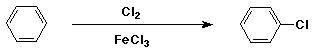
How much benzene is too much?
Brief exposure (5–10 minutes) to very high levels of benzene in air (10,000–20,000 ppm) can result in death. Lower levels (700–3,000 ppm) can cause drowsiness, dizziness, rapid heart rate, headaches, tremors, confusion, and unconsciousness.
How hazardous is benzene?
Benzene causes harmful effects on the bone marrow and can cause a decrease in red blood cells, leading to anemia. It can also cause excessive bleeding and can affect the immune system, increasing the chance for infection.
Are low levels of benzene dangerous?
Dec. 2, 2004 -- Gasoline, auto emissions, cigarette smoke: All contain benzene, a toxin whose chronic exposure, even at relatively low doses, has been linked to leukemia.
How much benzene does it take to cause cancer?
EPA estimates that 10 ppb benzene in drinking water that is consumed regularly or exposure to 0.4 ppb in air over a lifetime could cause a risk of one additional cancer case for every 100,000 exposed persons.
How much benzene is in a cigarette?
Smoking cigarettes with an average yield of 50 micron of benzene per cigarette has been compared with the occupational maximum exposure limit (16 mg m-3) concentration and with US studies on the home environment.
What is considered long term benzene exposure?
Long-term exposure of over a year or more to benzene is not safe. These effects can be devastating to the body and cause significant harm to an individual's blood. It can cause excessive bleeding, a significantly reduced and ineffective immune system and anemia.
How long does benzene stay in your system?
within 48 hoursBenzene is quickly eliminated from your system, usually within 48 hours. It is converted to products, medically known as metabolites, in the liver and bone marrow. Some of the harmful consequences of benzene exposure are caused by these metabolites.
What are four known health effects of benzene exposure?
Acute (short-term) inhalation exposure of humans to benzene may cause drowsiness, dizziness, headaches, as well as eye, skin, and respiratory tract irritation, and, at high levels, unconsciousness.
How do you test for benzene poisoning?
Blood counts of all components of the blood and examination of bone marrow are used to determine benzene exposure and its health effects. For people exposed to relatively high levels of benzene, complete blood analyses can be used to monitor possible changes related to exposure.
Can benzene cause brain tumors?
None of these studies indicated that benzene induced skin tumors; however, all possible tumor sites usually were not examined. EPA, IARC, and the Department of Health and Human Services have concluded that benzene is a human carcinogen.
Can benzene be absorbed through the skin?
Benzene is absorbed rapidly and extensively after inhalation and ingestion. It is absorbed less extensively through intact skin; however, percutaneous absorption may contribute to total body burden.
Should I be concerned about benzene?
Benzene is classified a Group A known human carcinogen by the U.S. Environmental Protection Agency. It's been most closely linked to leukemia and other blood cancers. Exposure to benzene can occur through the skin, as well as by inhalation or ingestion, according to the U.S. Food and Drug Administration.
What will happen if benzene comes in contact with your skin?
Dermal. Benzene can cause skin irritation and because it is a lipid solvent it degreases the skin, particularly after prolonged or repeated contact with the liquid. Locally, benzene can produce erythema, a burning sensation, and in more severe cases, edema and even blistering.
What PPE do you need for benzene?
What Personal Protective Equipment (PPE) is needed when working with benzene? Eye/Face Protection: Wear chemical safety goggles and face shield when contact is possible. Skin Protection: Wear chemical protective clothing e.g. gloves, aprons, boots.
Is benzene a poison?
Benzene is very poisonous. Poisoning can cause rapid death. However, deaths have occurred as long as 3 days after the poisoning.
What products have benzene in it?
Products Containing BenzenePaint, lacquer, and varnish removers.Industrial solvents.Gasoline and other fuels.Glues.Paints.Furniture wax.Detergents.Thinners.More items...•
What are the symptoms of high levels of benzene?
Death (at very high levels) Eating foods or drinking beverages containing high levels of benzene can cause the following symptoms within minutes to several hours: Vomiting. Irritation of the stomach. Dizziness. Sleepiness. Convulsions. Rapid or irregular heartbeat.
How does benzene affect the body?
(Long-term exposure means exposure of a year or more.) Benzene causes harmful effects on the bone marrow and can cause a decrease in red blood cells, leading to anemia.
How does benzene affect the immune system?
For example, it can cause bone marrow not to produce enough red blood cells, which can lead to anemia. Also, it can damage the immune system by changing blood levels of antibodies and causing the loss of white blood cells.
How is benzene poisoning treated?
How benzene poisoning is treated. Benzene poisoning is treated with supportive medical care in a hospital setting. No specific antidote exists for benzene poisoning. The most important thing is for victims to seek medical treatment as soon as possible.
How to get rid of benzene in your body?
Washing yourself. As quickly as possible, wash any benzene from your skin with large amounts of soap and water. Washing with soap and water will help protect people from any chemicals on their bodies. If your eyes are burning or your vision is blurred, rinse your eyes with plain water for 10 to 15 minutes.
How to protect yourself from benzene?
First, if the benzene was released into the air, get fresh air by leaving the area where the benzene was released. Moving to an area with fresh air is a good way to reduce the possibility of death from exposure to benzene in the air.
What is the source of benzene in the air?
How you could be exposed to benzene. Outdoor air contains low levels of benzene from tobacco smoke, gas stations, motor vehicle exhaust, and industrial emissions. Indoor air generally contains levels of benzene higher than those in outdoor air. The benzene in indoor air comes from products that contain benzene such as glues, paints, furniture wax, ...
How many people are exposed to benzene?
As many as 238,000 people may be occupationally exposed to benzene in the United States. These industries include benzene production (petrochemicals, petroleum refining, and coke and coal chemical manufacturing), rubber tire manufacturing, and storage or transport of benzene and petroleum products containing benzene.
How is the general population exposed to benzene?
Exposure of the general population to benzene mainly occurs through breathing air that contains benzene.
What is the metabolite of benzene?
Certain metabolites of benzene, such as phenol, muconic acid, and S-phenylmercapturic acid can be measured in the urine. The amount of phenol in urine has been used to check for benzene exposure in workers. The test is useful only when you are exposed to benzene in air at levels of 10 ppm or greater.
How does benzene get into the air?
Benzene can pass into air from water and soil surfaces. Once in the air, benzene reacts with other chemicals and breaks down within a few days. Benzene in the air can also be deposited on the ground by rain or snow. Benzene in water and soil breaks down more slowly.
How does benzene evaporate?
Benzene evaporates into air very quickly and dissolves slightly in water. Benzene is highly flammable. Most people can begin to smell benzene in air at approximately 60 parts of benzene per million parts of air (ppm) and recognize it as benzene at 100 ppm. Most people can begin to taste benzene in water at 0.5–4.5 ppm. One part per million is approximately equal to one drop in 40 gallons. Benzene is found in air, water, and soil. Benzene comes from both industrial and natural sources
What is the source of benzene in the air?
Tobacco smoke is another source of benzene in air, particularly indoors. Industrial discharge, disposal of products containing benzene, and gasoline leaks from underground storage tanks release benzene into water and soil. Benzene can pass into air from water and soil surfaces.
What is benzene used for?
Various industries use benzene to make other chemicals , such as styrene (for Styrofoam® and other plastics), cumene (for various resins), and cyclohexane (for nylon and synthetic fibers). Benzene is also used in the manufacturing of some types of rubbers, lubricants, dyes, detergents, drugs, and pesticides.
What is the safest level of benzene?
OSHA set the daily maximum safe level at 1 parts-per-million, or 1 ppm. That means 1 part benzene for every 1 million parts of air over the course of an 8-hour day. The short term exposure limit is 5 ppm for any 15-minute period. The U.S. Environmental Protection Agency sets the maximum benzene level in drinking water at 5 ppm.
How much benzene is in water?
The U.S. Environmental Protection Agency sets the maximum benzene level in drinking water at 5 ppm. Signs and symptoms of excessive benzene exposure — through either breathing it in or swallowing food and beverages containing it — can differ from one another. But symptoms are likely to show up within a few minutes to several hours after it happens, ...
How does benzene enter the body?
Benzene enters the body when you breathe it in or consume foods and drinks containing too much of the chemical. Source: U.S. Centers for Disease Control and Prevention, April 4, 2018. The U.S. Occupational Safety and Health Administration sets airborne benzene exposure limits for workplaces.
How long should you rinse benzene in your eyes?
If you get benzene in your eyes, rinse them for 10 to 15 minutes with water. Remove contact lenses and put them with the contaminated clothes.
How to reduce exposure to benzene?
There are several ways to reduce your exposure to benzene. Avoiding cigarette smoke is one of the most effective ways, as cigarettes are a major source of benzene. If you’re a smoker, quitting can reduce your exposure. Using gas stations with vapor recovery systems can protect you from exposure.
Why do we have to be exposed to benzene?
You may also be exposed in your home or workplace because benzene is used in paints, furniture wax and other common household items. But if you work in an industry or profession that uses benzene, you are likely to face much greater exposure.
Can benzene be detected in urine?
Testing & Treatment for Exposure. There are lab tests that can measure benzene levels in your blood, breath or urine. But they can only detect a recent exposure and cannot predict future health effects. Sudden exposure to high levels of benzene requires a hospital treatment.
What is the effect of benzene on the body?
The chemical quickly evaporates and the toxic fumes target the brain, heart, lungs, and other vital organs. Moreover, many researchers believe that benzene alters cellular DNA, which is why long term exposure to the chemical is associated with various serious illnesses that may also be fatal.
What is benzene used for?
Benzene is a petroleum-based industrial solvent that, until rather recently, was also used in after-shave lotions, decaffeinated coffee, unleaded gasoline, and consumer solvents like spot removers and paint strippers.
Why is benzene used in miscarriages?
Most likely because it causes genetic mutations, benzene causes aneuploid (irregular chromosome count) in male sperm. This condition is the leading cause of miscarriages. It is also linked to retarded fetal development and male fertility issues.
Why is benzene used in oil refineries?
Because it has such a high octane level, benzene is a mainstay at many oil refineries, because workers add this chemical to gasoline before it gets to the pump. The danger is significant not only for facility employees, but also for other people who live and work in the area, because even a tiny leak spews toxic fumes into the surrounding air.
Is benzene dangerous?
Benzene is one of the most commonly-used chemicals in the United States and also one of the most dangerous ones. For a free consultation with an experienced personal injury attorney in New York, contact Napoli Shkolnik PLLC. We do not charge upfront legal fees in negligence cases.
Does benzene cause leukemia?
Multiple laboratory tests have established that benzene triggers chromosomal changes in bone marrow, limiting its ability to produce red blood cells. These individuals not only are less able to fight off even low-grade infections, they are also more susceptible to leukemia.
Can benzene cause CML?
Doctors do not know what causes CML, but they do know that long term exposure to even low levels of benzene fumes is one of the leading risk factors . In fact, the most recent research indicates that there is a clear connection between the two.
How many hydrogen atoms are in benzene?
Benzene has 6 hydrogen atoms, fewer than the corresponding parent alkane, hexane, which has 14. Benzene and cyclohexane have a similar structure, only the ring of delocalized electrons and the loss of one hydrogen per carbon distinguishes it from cyclohexane. The molecule is planar.
Who discovered benzene?
Michael Faraday first isolated and identified benzene in 1825 from the oily residue derived from the production of illuminating gas, giving it the name bicarburet of hydrogen. In 1833, Eilhard Mitscherlich produced it by distilling benzoic acid (from gum benzoin) and lime. He gave the compound the name benzin.
How does benzene react with hydrogen?
Via hydrogenation, benzene and its derivatives convert to cyclohexane and derivatives. This reaction is achieved by the use of high pressures of hydrogen in the presence of heterogeneous catalysts, such as finely divided nickel. Whereas alkenes can be hydrogenated near room temperatures, benzene and related compounds are more reluctant substrates, requiring temperatures >100 °C. This reaction is practiced on a large scale industrially. In the absence of the catalyst, benzene is impervious to hydrogen. Hydrogenation cannot be stopped to give cyclohexene or cyclohexadienes as these are superior substrates. Birch reduction, a non catalytic process, however selectively hydrogenates benzene to the diene.
What are the four processes that contribute to the production of benzene?
Four chemical processes contribute to industrial benzene production: catalytic reforming, toluene hydrodealkylation, toluene disproportionation, and steam cracking. According to the ATSDR Toxicological Profile for benzene, between 1978 and 1981, catalytic reformates accounted for approximately 44–50% of the total U.S benzene production.
How is benzene separated from the other aromatics?
The aromatic products of the reaction are then separated from the reaction mixture (or reformate) by extraction with any one of a number of solvents, including diethylene glycol or sulfolane, and benzene is then separated from the other aromatics by distillation.
What was the main source of benzene?
For commercial use, until World War II, most benzene was obtained as a by-product of coke production (or "coke-oven light oil") for the steel industry. However, in the 1950s, increased demand for benzene, especially from the growing polymers industry, necessitated the production of benzene from petroleum.
What was the first industrial-scale production of benzene?
Four years later, Mansfield began the first industrial-scale production of benzene, based on the coal-tar method. Gradually, the sense developed among chemists that a number of substances were chemically related to benzene, comprising a diverse chemical family.
