
How many dozen covalent bonds in 8G CH4?
(Can you sense my millennial sarcasm(Continue reading) So number of covalent bonds in 8 g CH4 = 3.011× 10^23 × 4 = 1.204 × 10^24 Well, how many DOZEN covalent bonds in 6 methane molecules?
What is the percentage of polar bonds in CH4?
Does CH4 have polar bonds? The molecular mass of an organic compound is 78 and its percentage combination is 92.4% and 7.6%. What is the empirical formula and molecular formula of the compound?
How many covalent bonds do nonmetals form?
Nonmetals will readily form covalent bonds with other nonmetals in order to obtain stability, and can form anywhere between one to three covalent bonds with other nonmetals depending on how many valence electrons they posses. Although it is said that atoms share electrons w Why does a covalent bond occur?
Why do atoms covalent bond with each other?
Covalent bonding occurs when pairs of electrons are shared by atoms. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full electron shell. By sharing their outer most (valence) electrons, atoms can fill up their outer electron shell and gain stability.
Does CH4 have single covalent bonds?
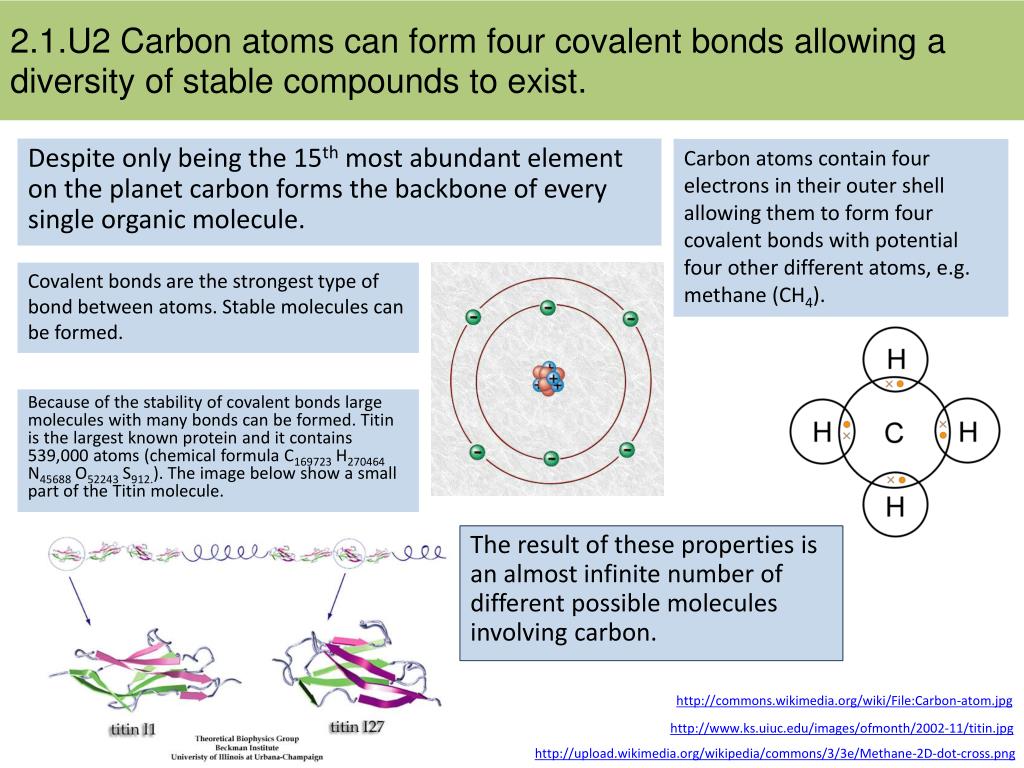
Methane (CH 4start text, end text, start subscript, 4, end subscript) is an example of a compound where non-polar covalent bonds are formed between two different atoms. One carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (H) atom.
Does CH4 have 4 single bonds?
Methane, CH4 , is the simplest type of alkane (hydrocarbon). It is saturated with four σ (single) bonds to hydrogen.
How many single bonds are present in CH?
four single bondsNote depiction of the four single bonds between the carbon and hydrogen atoms.
What bonds does CH4 have?
Methane, CH4, is a covalent compound with exactly 5 atoms that are linked by covalent bonds. We draw this covalent bonding as a Lewis structure (see diagram). The lines, or sticks, as we say, represent the covalent bonds. There are four bonds from a central carbon (C) linking or bonding it to four hydrogen atoms (H).
How many double bonds does CH4 have?
Conclusion. The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each.
How do you find the number of single bonds?
Calculation of Single bonds (A): where A = number of single bonds and Y is number of hydrogen atoms. E.g.: In C176H250, Y = 250, therefore A =[(3 x 250)/2] = 375 -2 = 373 single bonds.
What is a single covalent bond?
A single covalent bond can be defined as a chemical bond in which two atoms share two electrons to form one bond.
How many single bonds are present in nh3?
Therefore, it has three single bonds and a lone pair.
How many polar bonds does CH4 contain?
The molecule does not contain polar bonds. In addition, methane is overall non-polar and exists as a gas. We can calculate for the electronegativity difference to determine the ionic character of the C-H bond. The electronegativities of C and H are: C = 2.5.
What type of bond is CH4 polar or nonpolar?
CH4 is a nonpolar molecule as it has a symmetric tetrahedral geometrical shape with four identical C-H bonds. The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges to be almost zero.
Is C2H4 a double bond?
Ethylene, C2H4, is a colorless flammable gas and is the simplest alkene. It contains a double bond so it is an unsaturated hydrocarbon.
How many bonds are present in methane?
four singleThere are four single bonds present in methane as 4 hydrogen atom satisfies the carbon valency 4 which can be represented as shown in the figure.
How many covalent bonds are there in a water molecule?
No of covalent bonds in 1 water molecule = 2.
How many lone pairs does hydrogen have?
Taking another hydrogen results in the molecule having two lone pairs in total. The molecule will be similar to that of water. Hence, you've probably guessed it right, we subtract again by 2.5° to get an angle of 104.5°.
Why do lone pairs have smaller bond angles?
Evidently the lone pairs result in smaller bond angles due to stronger electrostatic repulsion.
Which pair of electrons is the strongest?
Basically the strongest is between lone pairs, then lone pair - bonded pair then lastly bonded pair-bonded pair.
Do compounds have atomic numbers?
Compounds do not have atomic numbers. Only elements have atomic numbers, which are the number of protons in the nuclei of their atoms. The atomic number of carbon (C) is 6, and the atomic number of hydrogen (H) is 1.
Is C H 4 a covalent compound?
A covalent bond is the sharing of valence electrons, and both carbon and hydrogen are nonmetals, therefore we may classify C H 4 as a covalent compound.
