How do hydrogen fuel cells work?
Photo of two hydrogen fuel cells. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. Fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is supplied.
How much power does a fuel cell produce?
The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure at which gases are supplied. A single fuel cell produces approximately 1 volt or less — barely enough electricity for even the smallest applications.
What is hydrogen and how does it work?
Hydrogen is a clean fuel that, when consumed in a fuel cell, produces only water. Hydrogen can be produced from a variety of domestic resources, such as natural gas, nuclear power, biomass, and renewable power like solar and wind. These qualities make it an attractive fuel option for transportation and electricity generation applications.
What is hydrogen fuel?
Hydrogen Fuel Basics Hydrogen is a clean fuel that, when consumed in a fuel cell, produces only water. Hydrogen can be produced from a variety of domestic resources, such as natural gas, nuclear power, biomass, and renewable power like solar and wind.

How much electricity does a hydrogen fuel cell produce?
approximately 1 voltA single fuel cell produces approximately 1 volt or less — barely enough electricity for even the smallest applications. To increase the amount of electricity generated, individual fuel cells are combined in series to form a stack.
What is the voltage output of hydrogen oxygen fuel cell?
-1.23VExplanation: The voltage output of hydrogen-oxygen fuel cell is -1.23V.
Do Hydrogen fuel cells produce electricity?
Like all-electric vehicles, fuel cell electric vehicles (FCEVs) use electricity to power an electric motor. In contrast to other electric vehicles, FCEVs produce electricity using a fuel cell powered by hydrogen, rather than drawing electricity from only a battery.
How much electricity can be produced from 1kg of hydrogen?
33.6 kWhIn electrical terms, the energy density of hydrogen is equal to 33.6 kWh of usable energy per kg, versus diesel which only holds about 12–14 kWh per kg. What this really means is that 1 kg of hydrogen, used in a fuel cell to power an electric motor, contains approximately the same energy as a gallon of diesel.
How much voltage can a fuel cell produce?
A typical hydrogen fuel cell produces 0.5 V to 0.8 V per cell. To increase the voltage individual cells can be connected in series.
How long can a hydrogen fuel cell last?
A full hydrogen tank will last around 300 miles (approx. 480 kilometers). Battery-powered cars can match this with very large batteries – which in turn will lead to an increase in both vehicle weight and charging times. temperature.
What is a disadvantage of using hydrogen fuel cells?
Expensive to manufacture due the high cost of catalysts (platinum) Lack of infrastructure to support the distribution of hydrogen. A lot of the currently available fuel cell technology is in the prototype stage and not yet validated. Hydrogen is expensive to produce and not widely available.
Can a normal car engine run on hydrogen?
Both hydrogen internal combustion engines and hydrogen fuel cells can power vehicles using hydrogen, a zero-carbon fuel. Hydrogen engines burn hydrogen in an internal combustion engine, in just the same way gasoline is used in an engine.
Why are hydrogen fuel cells not widely used?
5. Overall Cost. The cost for a unit of power from hydrogen fuel cells is currently greater than other energy sources, including solar panels. This may change as technology advances, but currently this cost is a barrier to widespread use of hydrogen even though it is more efficient once produced.
What is the cost of 1 kg hydrogen?
Current green hydrogen production costs range anywhere between ₹320 and ₹330 per kilogram in India. Green hydrogen costs in India could potentially fall by half to as low as ₹160-170 per kg by 2030, bringing parity with grey hydrogen and other fossil fuels, says a KPMG study.
Is hydrogen cheaper than diesel?
There are of course challenges to overcome: storing hydrogen is more complex and demanding than diesel, and while the cost per unit of a PEM fuel cell is broadly equivalent to the same capacity diesel generator, hydrogen is around three times more expensive as a fuel when all costs are factored in.
Is hydrogen more efficient than electric?
However, as hydrogen cars densely pack their energy storage, they're usually able to achieve longer distances. While most fully electric vehicles can travel between 100-200 miles on a single charge, hydrogen ones can get to 300 miles, according to AutomotiveTechnologies.
What happens when H2 and O2 react?
When molecular hydrogen (H2) and oxygen (O2) are combined and allowed to react together, energy is released and the molecules of hydrogen and oxygen can combine to form either water or hydrogen peroxide.
What is produced during H2 O2 fuel cell?
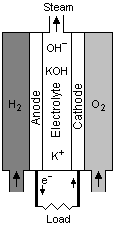
In a hydrogen oxygen fuel cell, electricity is produced. In this process H2(g) is oxidised at anode and O2(g) reduced at cathode. Given : Cathode: O2(g)+2H2O(l)+4e−→4OH−(aq) Anode : H2(g)+2OH−(aq)→2H2O(l)+2e− 4.48 L of H2 at 1 atm and 273 K is oxidised in 9650 sec.
What is the typical cell output voltage of a proton exchange fuel cell?
The operating voltage will be less, and at normal current densities such as 1 A/cm2, a typical cell voltage is 0.6 V.
What determines voltage of fuel cell?
A single fuel cell provides a voltage dependent on operating conditions such as temperature, applied load, and fuel/oxidant flow rates. The cell voltage drops due to several irreversible loss mechanisms (activation, ohmic, and concentration losses).
What is hydrogen fuel?
Hydrogen is a clean fuel that, when consumed in a fuel cell, produces only water. Hydrogen can be produced from a variety of domestic resources, such as natural gas, nuclear power, biomass, and renewable power like solar and wind.
How is hydrogen fuel made?
Today, hydrogen fuel can be produced through several methods. The most common methods today are natural gas reforming (a thermal process), and electrolysis. Other methods include solar-driven and biological processes.
What is the process of hydrogen?
Thermal processes for hydrogen production typically involve steam reforming, a high-temperature process in which steam reacts with a hydrocarbon fuel to produce hydrogen. Many hydrocarbon fuels can be reformed to produce hydrogen, including natural gas, diesel, renewable liquid fuels, gasified coal, or gasified biomass. Today, about 95% of all hydrogen is produced from steam reforming of natural gas.
How do biological processes produce hydrogen?
Biological processes use microbes such as bacteria and microalgae and can produce hydrogen through biological reactions. In microbial biomass conversion, the microbes break down organic matter like biomass or wastewater to produce hydrogen, while in photobiological processes the microbes use sunlight as the energy source.
What are the processes that are solar driven?
There are a few solar-driven processes, including photobiological, photoelectrochemical, and solar thermochemical. Photobiological processes use the natural photosynthetic activity of bacteria and green algae to produce hydrogen.
What can hydrogen fuel cells provide?
Photo of two hydrogen fuel cells. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices.
What is a direct methanol fuel cell?
The direct-methanol fuel cell (DMFC) is similar to the PEM cell in that it uses a proton conducting polymer membrane as an electrolyte. However, DMFCs use methanol directly on the anode, which eliminates the need for a fuel reformer.
What is phosphoric acid fuel cell?
Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°C. They are typically used in modules of 400 kW or greater and are being used for stationary power production in hotels, hospitals, grocery stores, and office buildings, where waste heat can also be used. Phosphoric acid can also be immobilized in polymer membranes, and fuel cells using these membranes are of interest for a variety of stationary power applications.
What is the difference between a fuel cell and a polymer electrolyte membrane?
A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. In a polymer electrolyte membrane fuel cell, a catalyst separates hydrogen atoms into protons and electrons, ...
What is a PEM fuel cell?
Polymer electrolyte membrane (PEM) fuel cells, also called proton exchange membrane fuel cells, use a proton-conducting polymer membrane as the electrolyte. Hydrogen is typically used as the fuel. These cells operate at relatively low temperatures and can quickly vary their output to meet shifting power demands.
What is the purpose of alkaline fuel cells?
Alkaline fuel cells use an alkaline electrolyte such as potassium hydroxide or an alkaline membrane that conducts hydroxide ions rather than protons. Originally used by the National Aeronautics and Space Administration (NASA) on space missions, alkaline fuel cells are now finding new applications, such as in portable power.
What is molten carbonate fuel cell?
Molten Carbonate Fuel Cells. Molten carbonate fuel cells use a molten carbonate salt immobilized in a porous matrix that conducts carbonate ions as their electrolyte. They are already being used in a variety of medium-to-large-scale stationary applications, where their high efficiency produces net energy savings.
What are the challenges of fuel cell technology?
Reducing cost and improving durability are the two most significant challenges to fuel cell commercialization. Fuel cell systems must be cost-competitive with, and perform as well or better than, traditional power technologies over the life of the system.
What is hydrogen used for?
Hydrogen is a versatile energy carrier that can be used to power nearly every end-use energy need. The fuel cell — an energy conversion device that can efficiently capture and use the power of hydrogen — is the key to making it happen.
What is the flow of electrons in a cell?
membrane to the other side of the cell, the stream of negatively-charged electrons follows an external circuit to the cathode. This flow of electrons is electricity that can be used to do work, such as power a motor.
