
How does sulfuric acid form and how it is made?
How does sulfuric acid form and how it is made? Sulfuric acid is produced from sulfur, oxygen and water via the contact process. In the first step, sulfur is burned to produce sulfur dioxide. This is then oxidised to sulfur trioxide using oxygen in the presence of a vanadium (V) oxide catalyst.
Is sulfuric acid a strong acid or base?
Sulfuric acid is also called the “king of chemicals or acid” because of its strong acid and more reactive nature. The presence of more H + ions in the solution defines sulfuric acid as a good conductor of electricity and a strong electrolyte.
What are the health hazards of sulfuric acid?
Sulfuric Acidis CORROSIVE and contact can severely irritate and burn the skin and eyes, and may lead to blindness. Inhaling Sulfuric Acidcan irritate the nose and throat. Sulfuric Acidcan irritate the lungs. Higher exposures may cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency.
What are the side effects of sulfuric acid?
What are the Causes of Sulfuric Acid Poisoning?
- Sulfuric Acid Poisoning is caused by the ingestion of sulfuric acid (liquid). The exposure may also be through direct skin or eye contact
- Inhalation of the corrosive sulfuric acid vapors may lead to respiratory problems
- This intake could be accidental, or in some cases intentional, to bring self-harm
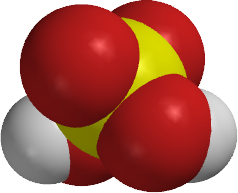
What produces sulfurous acid?
Sulfurous acid can be obtained by dissolving sulfur dioxide in water.
How is H2SO3 prepared?
Preparation: Sulfurous acid is prepared by dissolving sulfur dioxide in water. However, the reaction is reversible and the acid readily decomposes back into the reactants. Thus, sulfurous acid is not usually available in its acid form, but more commonly prepared as its sodium or potassium salts.
What is sulfuric acid and how is it formed?
Sulfuric acid is a constituent of acid rain, being formed by atmospheric oxidation of sulfur dioxide in the presence of water - i.e. oxidation of sulfurous acid. Sulfur dioxide is the main product when the sulphur in sulfur-containing fuels such as coal or oil is burned.
How is sulphurous acid prepared in laboratory?
definitionStep 1: Make sulphur dioxide.Step 2: Convert sulphur dioxide into sulphur trioxide (the reversible reaction at the heart of the process)Step 3: Convert sulphur trioxide into concentrated sulphuric acid.
Why is sulfurous acid a weak acid?
Unlike sulphuric acid (H2SO4), sulphurous acid (H2SO3) is a weak acid; that is, aqueous sulphurous acid acid does not dissociate entirely into H+ (H3O+) and bisulfite ions, meaning that the bisulfite ion is comparatively stronger in maintaining a proton when there is a base, such as water.
What is H2SO3 called?
Sulfurous acid | H2SO3 - PubChem.
Who produces sulfuric acid?
Sulfuric acid is one of the most important compounds made by the chemical industry....Annual production of sulfuric acid.World231 million tonnesChina74 million tonnesU.S.37 million tonnesIndia16 million tonnesRussia14 million tonnes1 more row
Where sulfuric acid is found?
Sulfuric acid is found in battery acid and in Earth's acid rain.
How was sulphuric acid first produced?
In the seventeenth century, German-Dutch alchemist and chemist Johann Glauber (1604−1670) combined sulfur with saltpeter (potassium nitrate) and heated the combination with steam to make sulfuric acid. Around 1735, sulfuric acid was first mass produced by English pharmacist Joshua Ward in glass containers.
What is sulfurous acid used for?
Aqueous solutions of sulfur dioxide, which sometimes are referred to as “sulfurous acid” are used as reducing agents and as disinfectants, as are solutions of bisulfite and sulfite salts. They are also mild bleaches, and are used for materials that may be damaged by chlorine-containing bleaches.
Does sulfuric acid occur naturally?
Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; for this reason, it is hygroscopic and readily absorbs water vapor from the air.
What is the pH of sulfurous acid?
The acidity of sulfurous acid is 1.5 on the pH scale.
What is the oxidation of H2SO3?
0:001:34How to find the Oxidation Number for S in H2SO3 (Sulfurous acid)YouTubeStart of suggested clipEnd of suggested clipThis is a neutral compound because there's no plus or minus sign up here all the oxidation numbersMoreThis is a neutral compound because there's no plus or minus sign up here all the oxidation numbers will add up to zero.
Is H2SO3 an acid or base?
Sulfurous acid, H2SO3, is a weak acid capable of providing two H+ ions (pKa1 = 1.9, pKa2 = 7.0).
Why is H2SO3 a double bond?
How many lone pairs and double bonds on sulfur atom according to the lewis structure of H2SO3? There is only one lone pair on sulfur atom in H2SO3 lewis structure. There is a one double bond between sulfur atom and oxygen atom.
Is H2SO3 a hydro acid?
Answer. H2SO3 is not a hydro acid.
What is sulphurous acid used for?
In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts an...
Is sulphurous acid a strong or weak acid?
Unlike sulphuric acid (H2SO4), sulphurous acid (H2SO3) is a weak acid; that is, aqueous sulphurous acid acid does not dissociate entirely into H+ (...
Is sulphurous acid dangerous?
Sulphuric acid can affect you by breathing in and moving through your skin. Sulphurous acid is a corrosive substance, so contact with potential eye...
Is Sulphur a Monoprotic acid?
No monoprotic acid does exist. There are two hydrogen protons in the starting state, sulphuric acid (H2SO4), In other words, the sulfate molecule w...
Is sulphurous acid soluble?
It is a colourless gas that has a high solubility in water. In solution, it hydrates to sulphuric acid (H2SO4), which in turn dissociates to form i...
1. Is Sulfurous Acid a Strong or Weak Acid?
Answer: Unlike sulfuric acid (H2SO4), the sulfurous acid (H2SO3) is defined as a weak acid; it means, aqueous sulfurous acid does not dissociate co...
2. What is Sulfurous Acid Used For?
Answer: The sulfuric acid is a dibasic, and solid acid. It reacts with bases to produce salts of sulfite and bisulfite.Its uses are given as: sulph...
3. Explain if Sulfur is a Monoprotic Acid?
Answer: None of the monoprotic acids exists. In the starting state, there exist two hydrogen protons, sulphuric acid (H2SO4). In other terms, the s...
4. Is sulfurous Acid Dangerous?
Answer: Sulfurous acid may affect us by getting inhaled and moving through the skin. Sulphurous acid is also a corrosive substance; hence, its cont...
What is sulfuric acid?
Sulfurous acid is also called Sulfur dioxide solution or dihydrogen trioxosulfate or trioxosulfuric acid. It is an intermediate species to produce acid rain from sulfur dioxide (SO 2 ).
What is sulphurous acid used for?
In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes.
Is Sulphur a Monoprotic acid?
No monoprotic acid does exist. There are two hydrogen protons in the starting state, sulphuric acid (H2SO4), In other words, the sulfate molecule was bound to certain acidic protons. Note also that monoprotic acids will contribute just one acidic proton, not two as in this acid.
Is sulphurous acid soluble?
It is a colourless gas that has a high solubility in water. In solution, it hydrates to sulphuric acid (H2SO4), which in turn dissociates to form ions of Bisulfite HSO 4–, and sulfite SO 32- ,.
Is sulphurous acid a weak acid?
Unlike sulphuric acid (H2SO4), sulphurous acid (H2SO3) is a weak acid; that is, aqueous sulphurous acid acid does not dissociate entirely into H+ (H3O+) and bisulfite ions, meaning that the bisulfite ion is comparatively stronger in maintaining a proton when there is a base, such as water.
Is sulfonic acid corrosive?
It is corrosive to tissue and metals. It is a sulfur oxoacid, tautomer of a sulfonic acid, and conjugate acid of a hydrogensulfite.
Is sulfur dioxide a corrosive gas?
It is a toxic, corrosive, and non-combustible compound. Inhaling, ingesting or skin contact with Sulfur dioxide solution causes severe injury which leads to death. In its molten form it can cause severe burns to eyes and skin. Therefore, avoid skin contact with this compound. On this compound it liberates corrosive, toxic and irritating gases.
What is sulfurous acid?
Read More. Sulfurous Acid is defined as a chemical compound having the chemical formula as H2SO3. H2SO3 is chemically known as Sulfurous Acid. Sulfurous acid is also referred to as Sulfur dioxide solution or trioxosulfuric acid or di-hydrogen trioxosulfate.
How does sulfuric acid affect the body?
Answer: Sulfurous acid may affect us by getting inhaled and moving through the skin. Sulphurous acid is also a corrosive substance; hence, its contact with eyes can seriously damage and irritate and cause a burning sensation. Sulfuric Acid breath can also irritate the throat and neck.
What is H2SO3 used for?
H2SO3 Uses (Sulfurous Acid) This compound is used as a reducing agent. Sulfurous acid can be used as an intermediate in industries. It is used in paper products manufacturing. It is also used as a disinfectant.
What is sulphuric acid used for?
Its uses are given as: sulphuric acid and its salts can be used as disinfectants and effective reducers. This is used as a mild bleaching agent in applications, where chlorine sensitive materials are included. 3.
What is the conjugate base of sulfur dioxide?
However, the conjugate bases of this elusive acid are common bisulfite (or hydrogen sulfite), anions, and sulfite. Whereas, weak and unstable acid, is formed when sulfur dioxide dissolves in water. The fact that sulfur dioxide compound actually exists in the solution cannot be explained exactly, but the molecules of this substance have been detected in the gaseous phase.
How many hydrogen protons are in H2SO4?
In the starting state, there exist two hydrogen protons, sulphuric acid (H2SO4). In other terms, the sulfate molecule is bound to particular acidic protons. It is also noted that monoprotic acids will contribute simply one acidic proton, not two as in this acid. 4.
What metals can oxidize?
The oxidizing acids which are mentioned above can react with the less reactive metals like copper, where the H+ cation is not reduced to hydrogen gas. For example, the reaction of dilute nitric acid with copper metal and its reaction is given below: 3 Cu + 8 HNO3+ → 3 Cu2+ + 2 NO + 4H2O + 6 NO3−.
How is sulfuric acid made?
The liquor is ordinarily made at the mill by burning sulfur to form SO 2 and dissolving this in water to produce sulfurous acid (H2 SO 3 ). Before sulfur is oxidized to form SO 2, it is handled above 160°C (320°F) because it is then a liquid and easy to convey. During combustion of sulfur at 1000°C (1830°F), the amount of oxygen must be carefully regulated to insure complete oxidation but not over oxidation that would result in the formation of sulfur trioxide (which forms sulfuric acid when added to water). The SO 2 gas is cooled fairly rapidly (if cooled too slowly, an undesired equilibrium reaction is favored with sulfur trioxide) before reaction with water. The sulfurous acid is then treated with alkaline earth element (as the hydroxide or carbonate salt) in an acid–base reaction. The reactions are summarized as follows (where M = Na, K, NH 4, ½Ca or ½Mg and are in solutions as their ions):
What is the name of the acid that forms sulfites?
Sulfurous acid forms both acid sulfites (also called bisulfites), such as NaHSO 3, sodium hydrogen sulfite, and normal sulfites, such as Na 2 SO 3, sodium sulfite. The best known and most soluble salts of sulfurous acid are those of the alkali metals, which are formed by reaction between sulfur dioxide and a solution of the alkali metal hydroxide. In essence, the reactions involve neutralization of sulfurous acid.
What is sulfur dioxide used for?
Aqueous solutions of sulfur dioxide, which sometimes are referred to as “sulfurous acid” are used as reducing agents and as disinfectants, as are solutions of bisulfite and sulfite salts. They are also mild bleaches, and are used for materials that may be damaged by chlorine-containing bleaches.
What is the CAS number for sulfurous acid?
Sulfurous Acid. Sulfurous acid has the formula H2 SO 3 and the molecular weight of 82.075 g/mol. Its CAS number is 7782-99-2. There is no clear evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase. The conjugate bases of this elusive acid are, however, common anions: bisulfite (or hydrogensulfite) ...
What is the name of the acid that dissolves in water to give solutions that contain sulfurous acid?
Sulfur dioxide dissolves in water to give solutions that contain sulfurous acid, H2 SO 3. The following equilibria are established by sulfurous acid, which is a weak diprotic acid known only in solution.
What is required for complete neutralization of the acid and the formation of the normal sulfite ion?
An excess of hydroxide ion is required for complete neutralization of the acid and the formation of the normal sulfite ion.
How to check for sulfuric acid in cooking liquor?
One can easily check for sulfuric acid in cooking liquor using TAPPI UM 600 to see if this is a problem at one's mill. It is accomplished by filtering the liquor, adding formaldehyde, adding BaCl 2 to precipitate sulfate as BaSO 4, settling overnight, filtering the precipitate, igniting the precipitate, and weighing the precipitate. UM 657 is analysis of sulfur burner gases for SO 2 and SO 3 by the Orsat apparatus.
How is sulfurous acid produced?
That is, it is produced when the sulfur dioxide reacts with the moisture present in the atmosphere.
What is sulfurous acid?
Sulfurous acid is a weak inorganic acid with the chemical formula H 2 S O 3. It is formed when sulfur dioxide is dissolved in water. Therefore, it is also referred to as the aqueous solution of sulfur dioxide. Sulfurous acid is found as an intermediate formed during the formation of acid rain (by the reaction of sulfur dioxide with the moisture present in the atmosphere). In this article, we are going to discuss more about sulfurous acid.
How many oxygen atoms are in sulfurous acid?
In the structure of sulfurous acid, the sulfur atom is in the centre, with the three oxygen atoms bonded to it. Out of the three oxygen atoms, two are single-bonded and one is double-bonded. The two hydrogen atoms are attached to the single-bonded oxygen atoms.
What is sulfur dioxide used for?
Sulfurous acid, which is also known as the aqueous solution of sulfur dioxide, is used as a disinfectant and as a reducing agent.
What is the boiling point of sulfurous acid?
The boiling point of sulfurous acid is approximately equal to – 60 ∘ C.
When sodium sulfate is added to an aqueous solution of sulfur dioxide, sodium bisulfit?
When sodium sulfate is added to an aqueous solution of sulfur dioxide, sodium bisulfite salt and sulfur dioxide are formed. The chemical equation for the reaction can be given as,
Is sulfurous acid a compound?
Sulfurous acid has not been detected or isolated as a separate compound. Also, it does not appear to exist in solution either. However, the spectroscopic evidence provides the following information: In the solution of sulfur dioxide in water, the spectroscopic evidence provided only the signals of S O 2 molecule and the bisulfite ( H S O 3 –) ion. The intensities of such signals were consistent with the following equilibrium.
Is sulfurous acid a tautomer?
Sulfurous acid is a sulfur oxoacid. It is a conjugate acid of a hydrogensulfite. It is a tautomer of a sulfonic acid.
Is sulfur corrosive or toxic?
Colorless, corrosive, moderately toxic liquid. When heated to decomposition it emits toxic fumes of oxides of sulfur [Lewis, 3rd ed., 1993, p. 1196].
What is the formula for sulfurous acid?
Sulfurous Acid Chemical Formula. The formula of the sulphurous acid is given as H 2 SO 3 where the molecule consists of two atoms of hydrogen, three atoms of oxygen and one atom of sulfur. Formula.
How many oxygen atoms are in sulfurous acid?
Looking at the chemical structure of acid we can see that the molecule has the sulfur atom in the middle and three oxygen atoms are bonded where two oxygen atoms are formed by a single-bond and one has a double-bond. The two hydrogen atoms are connected by a single bond to the oxygen atoms. The sulfurous acid structural formula is normally written as;
Is sulfurous acid a dibasic?
The sulphurous or sulfur ous acid formula should not be confused with the formula of sulphuric acid. While the formula may look similar but one of the main differences is that there is less number of oxygen atoms in sulfurous acid. Their physical and chemical properties also differ to some extent. Sulphurou s acid is typically a dibasic ...
