How did Proust discover the law of definite proportions?
Proust studied copper carbonate, the two tin oxides, and the two iron sulfides to prove this law. He did this by making artificial copper carbonate and comparing it to natural copper carbonate. With this he showed that each had the same proportion of weights between the three elements involved .
When was the law of definite proportions discovered?
Law of Definite Proportions (Jeremias Benjamin Richter) Evidence for the existence of atoms was the law of definite proportions proposed by Jeremias Benjamin Richter in 1792. Richter found that the ratio by weight of the compounds consumed in a chemical reaction was always the same.
How was the law of multiple proportions discovered?
In 1792, Bertrand Pelletier discovered that a certain amount of tin will combine with a certain amount of oxygen to form one tin oxide, or twice the amount of oxygen to form a different oxide.
Who discovered the theory of proportion?
Euclid (c. 300 BC) summarized two theories of proportion: one for magnitudes in geometry—the continuum of real numbers in today's terminology (Heath, 1923, pages 112-186: Euclid, BookV); and one for multitudes in arithmetic—discrete, natural numbers (Heath, 1923, pages 275-344: Euclid, Book VII).
Who proposed definite proportions?
A French scientist named Joseph Proust conducted experiments that demonstrated this law, from the late 1700's to the early 1800's. The Law of Definite Proportions is also sometimes called Proust's Law.
How does Dalton's theory explain the law of definite proportions?
The Dalton atomic theory explains the law of definite proportions. Dalton proposed that the smallest particle of carbon monoxide is a molecule which contains one oxygen atom and one carbon atom. When oxygen atom contains mass about 1.33 times the carbon atom, carbon monoxide will have the above composition.
Who was the first to introduce the law of changing proportions?
History. The law of constant proportion was given by Joseph Proust in 1797. This observation was first made by the English theologian and chemist Joseph Priestley, and Antoine Lavoisier, a French nobleman and chemist centered on the process of combustion.
What is Dalton's law of definite proportions?
Law of Definite Proportions states that in a given type of chemical substance, the elements are always combined in the same proportions by mass. The Law of Definite Proportions applies when elements are reacted together to form the same product.
When did Dalton proposed law of multiple proportions?
1803John Dalton (1803) stated, "'When two elements combine with each other to form two or more compounds, the ratios of the masses of one element that combines with the fixed mass of the other are simple whole numbers'.
What theory did John Dalton formulate?
Summary. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Dalton based his theory on the law of conservation of mass and the law of constant composition. The first part of his theory states that all matter is made of atoms, which are indivisible.
How did the law of multiple proportions encourage Dalton to introduce an atomic theory?
How did the law of multiple proportions encourage Dalton to introduce atomic theory? In his work, Dalton found that the weight ratio of the element is always whole number multiple ratio and this is due to repetition of the atoms of the same element.
Who created the law of definite proportions?
The law of definite proportions contributed to, and was placed on a firm theoretical basis by, the atomic theory that John Dalton promoted beginning in 1803, which explained matter as consisting of discrete atoms, that there was one type of atom for each element, and that the compounds were made of combinations of different types of atoms in fixed proportions.
Which law forms the basis of stoichiometry?
Along with the law of multiple proportions, the law of definite proportions forms the basis of stoichiometry.
What is the law of constant composition?
In chemistry, the law of definite proportion, sometimes called Proust's law, or law of constant composition states that a given chemical compound always contains its component elements in fixed ratio (by mass) and does not depend on its source and method of preparation.
Is the law of definite proportions a novel idea?
The law of definite proportions might seem obvious to the modern chemist, inherent in the very definition of a chemical compound. At the end of the 18th century, however, when the concept of a chemical compound had not yet been fully developed, the law was novel. In fact, when first proposed, it was a controversial statement ...
Does isotopic composition affect mass?
In addition, the isotopic composition of an element can vary depending on its source, hence its contribution to the mass of even a pure stoichiometric compound may vary. This variation is used in radiometric dating since astronomical, atmospheric, oceanic, crustal and deep Earth processes may concentrate some environmental isotopes preferentially. With the exception of hydrogen and its isotopes, the effect is usually small, but is measurable with modern day instrumentation.
What is the law of definite proportions?
The law of definite proportions states samples of a compound will always contain the same proportion of elements by mass. The mass ratio of elements is fixed no matter where the elements came from, how the compound is prepared, or any other factor. Essentially, the law is based on the fact that an atom of a particular element is the same as any other atom of that element. So, an atom of oxygen is the same, whether it comes from silica or oxygen in the air.
Who was the first person to discover the law of combustion?
French chemist Joseph Proust (1754–1826) is credited with the discovery, but English chemist and theologian Joseph Priestly (1783–1804) and French chemist Antoine Lavoisier (1771– 1794) were the first to publish the law as a scientific proposal in 1794, based on the study of combustion. They noted metals always combine with two proportions of oxygen.
What is the formula for wustite?
The ideal formula for iron oxide is FeO, but the crystal structure is such that there are variations. The formula for wustite is written Fe 0.95 O.
What is the law of definite proportions?
The law of definite proportions states that a chemically pure substance always contains the same set of elements combined together in a definite proportion by weight. In other words, the law can be stated as the percentage composition of an element in a compound is always fixed.
Why is the law of definite proportions important?
The law of definite proportions is crucial in analytical chemistry and forms the basis of various other chemical discoveries. The law explains that the chemical composition of a chemically pure substance is the same irrespective of the source and the elements in the compound exist in the same ratio by mass. There has been a correlation of the law ...
Who is Joseph Proust?
Joseph Louis Proust (26 September 1754- 5 July 1826) was a French chemist who is best known for the discovery of the law of definite proportions or the law of constant composition.
Why does isotopic composition vary?
This is due to fluctuations in the mass ratios of the elements.
Does the law of definite proportions hold true for all chemical compounds?
Even though the law of definite proportions is a building block of modern analytical chemistry, the law doesn’t hold true for all chemical compounds. Some of the exceptions to this law are;
Does water have the same ratio?
The ratio of the elements in water remains the same even if the weight or moles of water are increased. Water present in any state of matter contains the elements in the same proportion as the law of definite proportion holds true for water.
Is the law of definite proportions valid for polymers?
The law of definite proportions holds true for all molecules in the gaseous state and for smaller molecular compounds in other states of matter, but it is not valid for polymers. The polymers with even identical monomers have considerable variation in the polymer molecule length, branching, termination, etc.
What is the law of definite proportions and how does it apply to this experiment?
1 Answer. Ernest Z. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. … To tell if compounds containing the same elements are the same or different, we can determine the ratios of their masses.
What is Dalton’s Law of definite proportions?
The law of definite proportions states that a chemical compound always contains exactly the same proportion of elements by mass. … The atomic theory explains the law of definite proportions: Dalton proposed that the smallest particle of carbon monoxide was a molecule.
What does the law of definite composition mean?
Logic. the law that either a proposition or its denial must be true .
What is the meaning of constant composition?
The law of constant composition states that in a given chemical compound, all samples are made up of the same elements combined in the same proportions.
What is the difference between the law of definite proportions and multiple proportions?
The law of multiple proportions is an extension of the law of definite composition, which states that compounds will consist of defined ratios of elements.
What is law of constant proportion Class 9?
The law of constant proportions states that chemical compounds are made up of elements that are present in a fixed ratio by mass. This implies that any pure sample of a compound, no matter the source, will always consist of the same elements that are present in the same ratio by mass.
Do mixtures have definite properties?
Mixtures are unlike chemical compounds, because: … Mixtures have variable compositions, while compounds have a fixed, definite formula. When mixed, individual substances keep their properties in a mixture, while if they form a compound their properties can change.
What is meant by the law of definite proportions?
Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent elements. …
What is the law of definite proportions for kids?
The law of definite proportions (or Proust’s law) states that a chemical compound always has exactly the same proportion of elements by mass. … This states that all samples of a given chemical compound have the same elemental composition by mass.
Why is the law of definite composition important?
The law of definite proportions dictates that a name is always associated with a specific ratio of elements found in a chemical compound. If the ratio of elements is different from that specific ratio then it is not the same compound and therefor has a different name.
What is law of constant proportion Class 9?
The law of constant proportions states that chemical compounds are made up of elements that are present in a fixed ratio by mass. This implies that any pure sample of a compound, no matter the source, will always consist of the same elements that are present in the same ratio by mass.
What is the difference between the law of definite proportions and multiple proportions?
The law of multiple proportions is an extension of the law of definite composition, which states that compounds will consist of defined ratios of elements.
What is law of multiple proportions explain with examples?
This example illustrates the law of multiple proportions: Whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers. … Carbon can form two different compounds with oxygen.
What does it mean to have a definite composition?
Law of definite composition states that the elements in a given compound are always combined in the same proportion by mass. This law form the basis for the definition of a chemical compound.

Law of Definite Proportions Definition
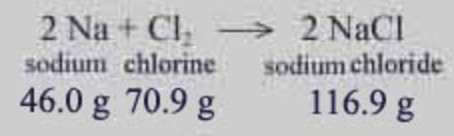
Law of Definition Proportions Example
The law of constant proportion was given by Joseph Proust in 1797. This observation was first made by the English theologian and chemist Joseph Priestley, and Antoine Lavoisier, a French nobleman and chemist centered on the process of combustion.
I shall conclude by deducing from these experiments the principle I have established at the commencement of this memoir, viz. that iron like many other metals is subject to the law of nat…
History of The Law of Definite Proportions
Exceptions to The Law of Definite Proportions