
How to do an osmosis experiment in potatoes?
Osmosis in potatoes. Cylinders or discs of fresh potato are often used to investigate osmosis in living cells. To carry out this type of experiment, you need to: The percentage change in mass can be calculated for each piece of potato. Example one. A piece of potato has a mass of 2.5 g at the start and 3.0 g at the end.
How do you demonstrate the process of osmosis?
The process of osmosis may be demonstrated by the simple osmometer which is the thistle funnel experiment. Demonstration of osmosis in a living system can be one using the potato osmoscope. A potato is peeled and one side is flattened which serves as the base.
What happens when water is added to a potato?
Therefore, water moves from the cells of the potato to the surrounding hypertonic solution in the beaker through osmosis (Kurzweil & Walker, 2009). The movement of water through the process of osmosis into the hypertonic solution results in the decrease in the mass of the potato strips after 45 minutes.
What does the minus sign mean in osmosis in potatoes?
It will have gained water by osmosis. A piece of potato has a mass of 2.5 g at the start and 2.0 g at the end. The minus sign shows that it has lost mass. It will have lost water by osmosis. Rachel carried out an experiment to investigate osmosis in potatoes.
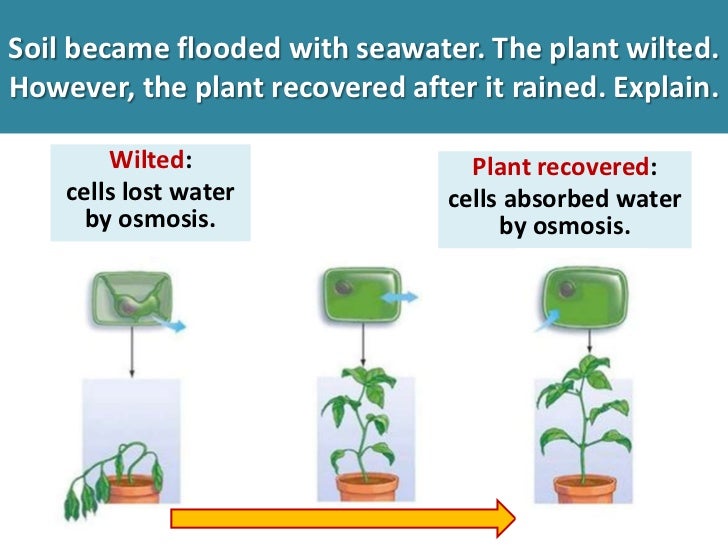
How will you demonstrate osmosis using a potato?
ProcedureSlice the potato tuber into two equal halves with the help of a scalpel or a blade. ... From the mid-region of the tuber, scoop from the soft parenchyma, so as to form a tiny cavity of a square or a circular shape. ... Fill up half the cavity with the freshly prepared 20% sugar solution.More items...•
How does osmosis happen in potatoes?
Water will move from an area of less salt to more salt (more water to less water), and so when the potato is placed in the saltwater, all the water that is inside the potato (yes, plants have a lot of water inside of them, that's what gives a plant it's structure) moves out by osmosis.
Why do we use potatoes to test osmosis?
Osmosis, the process in which solvent molecules move from an area of lower solute concentration to an area of higher solute concentration, can easily be demonstrated with potato experiments. Potatoes are full of both water and starch, and will gain water when immersed in watery solutions.
What happens when you put a potato in water?
Growing Potatoes in Water The submerged portion of the potato will absorb water and act as a nutrient source for the growing plant, eventually developing roots under the water as well. A potato vine will begin growing from the sprouted eye as well.
What is the conclusion for the osmosis potato lab?
Answer: The concept of osmosis is clearly demonstrated by this experiment. Water molecules are observed to have moved from the region where they are highly concentrated to the region where they have a low concentration through a semi-permeable membrane in the cells of the potato.
Why do potatoes absorb water?
When you bake a potato, the starch granules absorb the moisture within the potato. Within the confines of the potato skin, moisture soon turns to steam that expands with great force, separating the starch granules and making a fluffy baked potato.
What is the procedure of osmosis?
Osmosis is a process of movement of solvents through a semi-permeable membrane from a region of lower solute concentration to higher solute concentration. On the contrary, diffusion does not require a semi-permeable membrane to occur and the molecules move from a region of higher concentration to lower concentration.
What happens when you put a potato in salt water?
The potato in the salt water shrinks because water moves from the potato into the more concentrated salt water. In contrast, water moves from the less concentrated distilled water into the potato causing it to expand.
Why do potatoes absorb water?
When you bake a potato, the starch granules absorb the moisture within the potato. Within the confines of the potato skin, moisture soon turns to steam that expands with great force, separating the starch granules and making a fluffy baked potato.
What happens to potatoes in salt water?
The potato in the salt water shrinks because water moves from the potato into the more concentrated salt water. In contrast, water moves from the less concentrated distilled water into the potato causing it to expand.
What happens when you soak potatoes in salt water?
Soaking potatoes in salt water draws out water and removes excess starch, ensuring a crisper, firmer result when cooking. Soak peeled, chopped potatoes for fries in salt water for 15 minutes to 24 hours – the longer, the better.
What happens when a potato is placed in a hypertonic solution?
Osmosis can be seen very effectively when potato slices are added to a high concentration of salt solution (hypertonic). The water from inside the potato moves out of the potato cells to the salt solution, which causes the potato cells to lose turgor pressure.
How long should a potato osmometer stay in water?from byjus.com
The set up should remain uninterrupted for close to 1 hour.
How does osmosis affect the environment?from byjus.com
Osmosis maintains cell turgidity. It causes the transportation of nutrients and discharge of metabolic waste products. It preserves the interior environment of a living entity to maintain an equilibrium between the intracellular fluid levels and water.
What is plasmolysis in plants?from byjus.com
It is a process, wherein the protoplasm of the plant cell turns round as a result of contraction when placed in a hypertonic solution due to exosmosis resulting in the decline in the tension of the cell wall. Q.2.
Why does the sucrose level increase in the osmometer?from byjus.com
An increase in the level of sucrose solution is observed in the osmometer. It is because of the entrance of water due to endosmosis from the beaker. Also, a water potential gradient is built between the sucrose solution in the external water and the osmometer.
What is the movement of molecules from a region of higher concentration to a region of lower concentration?from byjus.com
A.3. The movement of molecules from a region of higher concentration to a region of lower concentration. Osmosis is a type of diffusion.
What is the movement of water from a region of higher water potential to a region of lower water potential?from byjus.com
In other words, osmosis is the diffusion or movement of water from a region of higher water potential to a region of lower water potential.
What is the process of a solvent passing through a semi-permeable membrane?from byjus.com
Osmosis is the phenomena in which solvent molecules pass through a semi-permeable membrane from an area of higher concentration to an area of lower concentration. The process continues until the quantity of fluid is balanced or equalized in both regions, the region of higher concentration and the region of lower concentration ...
What is the key to understanding the issue of osmosis?from metrofamilymagazine.com
Osmosis is the key to understanding this issue. Osmosis is the diffusion of water across a semi-permeable membrane (yikes!) from an area of high concentration of water, to an area of low concentration.
How does water move out of a potato?from metrofamilymagazine.com
Water will move from an area of less salt to more salt (more water to less water), and so when the potato is placed in the saltwater, all the water that is inside the potato (yes, plants have a lot of water inside of them, that’s what gives a plant it’s structure) moves out by osmosis.
How is a plasmolyzed cell made turgid?from grin.com
A plasmolyzed cell can be made turgid by immersing it in distilled water for some time (deplasmolysis) (Odom et al, 2017). Therefore for osmosis to occur, the two solutions must be separated by a semi-permeable, membrane, for this case, the potato cell membrane is only permeable to water molecules from potato cell but is not permeable ...
What is the force created by osmosis?from grin.com
As osmosis is taking place, a force will be created in the hypertonic solution that aims to prevent osmosis from taking place; such a force is called osmotic pressure. If osmotic pressure of a solution is high, it will draw a lot of water from the adjacent solution, and if it is low, it will draw little waiter.
What is the spontaneous net movement of solvent molecules through a partially permeable membrane into a region of higher so?from phdessay.com
Introduction: Osmosis is the spontaneous net movement of solvent molecules through a partially permeable membrane into a region of higher solute concentration in the presence of water. An isotonic solution is one in which the concentration of solutes is the same inside the cell as outside the cell (STAYS THE SAME).
What is the process where a plane cell loses water, shrinks and becomes flaccid?from grin.com
The process where a plane cell loses water, shrink and become flaccid is called deplasmolysis.
What is hypertonic solution?from phdessay.com
A hypertonic solution will have a higher concentration of solutes than the cell and will have a higher osmotic pressure outside the cell than inside the cell. (SHRINKS) The objective of this experiment was to study the effects of one concentrated solution (with different percentages of solute) on white and sweet potatoes.

Introduction
The Aim of The Experiment
Variables
Hypothesis
Equipment
Preparing Sucrose Solutions of Different Concentrations
Preparing The Strips
Placing The Potato Cylinders in The Different Sucrose Concentration Solutions
- The potato strips were then carefully bloated using tissue paper without squeezing them to remove water from the external part. Their respective weights were measured and noted. Subsequently, they were placed in the prepared sucrose solutions. After forty-five minutes the strips were removed, bloated, and reweighed.
Results
Discussion and Conclusion