
How to Make the Solution
- Put 1 teaspoon of calcium hydroxide in a clean glass jar, up to 1 gallon in size. ...
- Fill the jar with distilled or tap water.
- Shake the jar vigorously for 1-2 minutes, then let it stand for 24 hours.
- Being careful not to stir up the sediment, pour the clearer solution off the top of the jar through a clean coffee filter or filter paper.
What happens when you mix calcium hydroxide and water?
In the following demonstration, a chunk of calcium metal is dropped into a beaker of distilled water. After a second or so, the calcium metal begins to bubble vigorously as it reacts with the water, producing hydrogen gas, and a cloudy white precipitate of calcium hydroxide.
How do you calculate the Ksp for Ca(OH)2?
The equation for the Ksp of Ca(OH)2 is the concentration [Ca2+] times the concentration [OH-] taken to the second power, since the OH- has a coefficient of 2 in the balanced equation. Plug the concentrations of each of the products into the equation to calculate the value of Ksp.
What are the uses of calcium hydroxide?
Uses of Calcium Hydroxide
- In the process of sewage treatment, calcium hydroxide is used as a clarifying agent or as a flocculant.
- Ca (OH) 2 is used in the paper industry during the Kraft process of converting wood into wood pulp.
- It is a very important compound in the preparation of ammonia.
- This compound is also used as a pH modifier due to its basicity.
Is Ca OH 2 soluble?
Is Ca(OH)2 (Calcium hydroxide) soluble or insoluble in water ? The answer is that it is slightly soluble in water. Although it is an ionic compound, it does ...
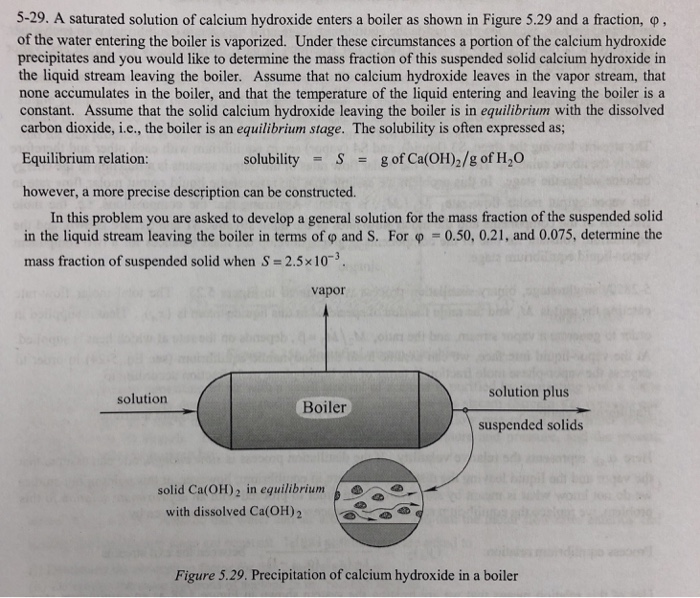
What is saturated solution of calcium hydroxide?
Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide.
What is the concentration of saturated calcium hydroxide?
about 0.0203 Mthe saturated solution is about 0.0203 M and has a pH of 12.45 at 25° C.
How do you make a calcium hydroxide solution?
0:213:37Make Calcium Hydroxide - YouTubeYouTubeStart of suggested clipEnd of suggested clipFirst weigh out your reactants you can use sixty grams of calcium chloride 240 grams of sodiumMoreFirst weigh out your reactants you can use sixty grams of calcium chloride 240 grams of sodium hydroxide. This will give a slight excess of calcium chloride in. Solution. Add some water to the calcium
What is the solution of Ca OH 2?
Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca(OH)2. It is an inorganic compound which has a white, powdery appearance in its solid-state....Properties of Calcium Hydroxide.Chemical FormulaCa(OH)2Melting Point853 K4 more rows•Aug 20, 2020
What is a saturated solution?
A solution in which the maximum amount of solvent has been dissolved. Any more solute added will sit as crystals on the bottom of the container.
How do you find the concentration of calcium hydroxide?
Worked exampleAmount of calcium hydroxide = 7.4 ÷ 74 = 0.1 mol.Volume of water = 250 ÷ 1000 = 0.25 dm 3Concentration = 0.1 ÷ 0.25 = 0.4 mol/dm 3
How do you make saturated lime water?
Put 1 teaspoon of calcium hydroxide in a clean glass jar, up to 1 gallon in size. (Limewater is a saturated solution, which means there will be some extra chemical that doesn't dissolve. A teaspoon will result in a fully saturated solution whether you use a gallon jar or a smaller one.)
What is the balanced equation for Cao H2O Ca OH 2 H2?
Search by reactants (Ca, H 2O) and by products (Ca(OH) 2, H 2)1H2O + Ca → H2 + Ca(OH)22H2O + O2 + Ca → H2 + Ca(OH)2
What happens when calcium hydroxide is heated?
Calcium hydroxide decomposes to produce calcium oxide and water. However, lithium hydroxide does not decompose upon heating. Some unstable acids decompose to produce nonmetal oxides and water.
What is the pH of a saturated solution of Ca OH 2?
9pH of saturated solution of Ca(OH)2 is 9 .
How is Ca OH 2 produced?
Calcium hydroxide, also called slaked lime, Ca(OH)2, is obtained by the action of water on calcium oxide. When mixed with water, a small proportion of it dissolves, forming a solution known as limewater, the rest remaining as a suspension called milk of lime.
What is the pH of calcium hydroxide?
Estrela and Figueiredo13 state that calcium hydroxide is a white alkaline (pH 12.8) powder with poor solubility in water (solubility of 1.2 g.L–1 of water at 25 °C). It is a strong base obtained by calcining calcium carbonate until it transforms into calcium oxide (quicklime).
How do you determine concentration?
The standard formula is C = m/V, where C is the concentration, m is the mass of the solute dissolved, and V is the total volume of the solution. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow.
What is the solubility product of Ca OH 2?
4.42×10−5Q. The solubility product (Ksp) of Ca(OH)2 at 25∘C is 4.42×10−5.
What is the solubility of Ca OH 2?
WaterCalcium hydroxide / Soluble inWater is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms. It is vital for all known forms of life, even though it provides neither food, energy, nor organic micronutrients. Wikipedia
What is the molar solubility of Ca OH 2?
. Calculate the molar solubility. 3) The solubility of Ca(OH)2 is found to be 0.233 g/L.
How to determine the concentration of dissolved hydroxide?
The concentration of dissolved hydroxide will be determined by acid-base titration with standardized HCl solution. The
How much liquid above precipitate to TA?
If your TA prepares the saturated Ca (OH) 2 solution, carefully dispense approximately 30 mL of the liquid above the precipitate into an Erlenmeyer flask and stopper it immediately. PLEASE be sure to replace the lid on the reagent container (the TA-prepared solution). Proceed to step 5.
How to separate fine particles from supernatant?
To better separate the fine precipitate particles from the supernatant, you may use two pieces of filter paper or you may filter the solution twice. Filtration may take a long time, so make the dilutions for the rest of the experiment while you wait. Do not use vacuum filtration. Do not wash the precipitate. 6.
Can lime water react with carbon dioxide?
Once the filtration is complete, immediately stopper the flask containing the supernatant. Limewater can react with carbon dioxide to yield the very insoluble calcium carbonate.
Is calcium metal a reducing agent?
Calcium metal is a strong reducing agent and should not touch skin. You may have to score the metal to rub off the tarnish and expose a clean surface to get the reaction to start. You should see the white precipitate of solid Ca (OH)2, indicating that the supernatant liquid is a saturated solution of limewater.
How to make a saturated solution of calcium hydroxide?
The method involves mixing distilled water with calcium hydroxide and shaking the mixture thoroughly , making a saturated solution of calcium hydroxide.
How to make calcium hydroxide?
1 teaspoon of calcium hydroxide (slaked lime) ½ Jar of Water. Steps to Prepare. Pour in 1 teaspoon of slaked lime into a jar filled with water and place a cover on the jar. Shake it thoroughly. At first, shake for a minute or two and then allow the mixture to stand for 24 hours.
What is the reaction of calcium hydroxide and carbon dioxide?
Calcium hydroxide in lime water reacts with carbon dioxide to give calcium carbonate which forms an insoluble suspension in the solution. This property makes limewater useful in detecting the presence of carbon dioxide. A simple experiment that demonstrates this reaction is to exhale into limewater and observe the change in its color.
Why is calcium hydroxide milky?
If excess calcium hydroxide is added, the solution has a milky appearance due to suspended calcium hydroxide particles.
How much calcium hydroxide can dissolve in water?
Calcium hydroxide is less soluble in comparison to barium hydroxide. 1 liter of pure water can dissolve about 1 gram of calcium hydroxide.
What is added to reef tanks to restore calcium?
Organisms in reef tanks consume calcium from water. Limewater is added to the tanks to restore the lost calcium.
