Normal saline (0.9% sodium chloride) contains 308 mOsm/L and is considered isotonic. In contrast, 0.45% sodium chloride (154 mOsm/L) and 0.225% sodium chloride (77 mOsm/L) are hypotonic. What are the benefits of sodium chloride?
What is the percent isotonic of sodium chloride?
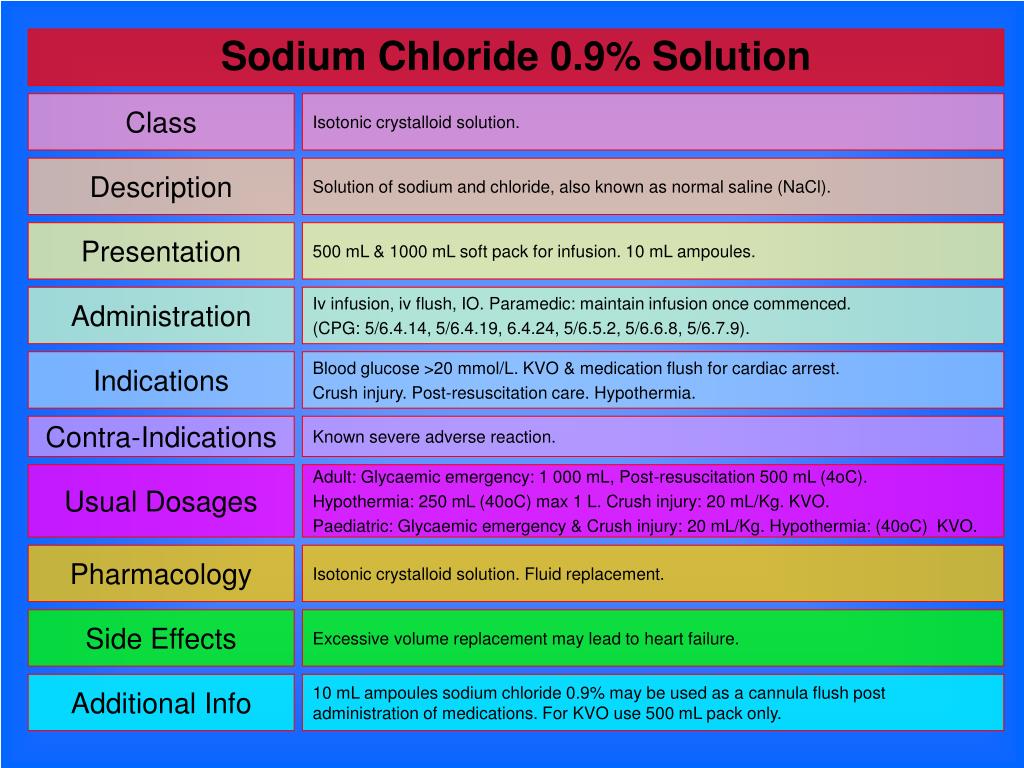
Sodium chloride solution 0.9% isotonic. Sodium Chloride 0.9% solution is a substance with de-toxication and rehydration properties. Indications of the sodium chloride solution 0.9% isotonic. This drug is used when a person loses a large amount of liquid outside the cells.
What are the different types of hypotonic solutions?
Types of Hypotonic Solutions: 1 45% sodium chloride (0.45% NaCl) 2 33% sodium chloride 3 2% sodium chloride 4 5% dextrose in water
What is the percentage of sodium chloride in blood cells?
Blood cells are isotonic with 0.9 % sodium chloride solution. What happens if we place blood cells in a solution containing: a) 1.2 % sodium chloride solution?
How does hypotonic solution affect the body?
Hypotonic solution hydrate the cells, but causes fluid depletion in the circulatory system. (Fluid shift from intravascular space to intracellular and interstitial spaces.) Hypotonic solutions lower serum sodium levels so it’s essential to monitor sodium levels.

Is 0.9 sodium chloride an isotonic solution?
Sodium Chloride 0.9% intravenous infusion is an isotonic solution, with an approximate osmolarity of 308 mOsm/l.
What is 9% sodium chloride?
0.9% Sodium Chloride Injection, USP is sterile and nonpyrogenic. It is a parenteral solution containing sodium chloride in water for injection intended for intravenous administration. 308 mOsmol/L (calc.). The pH is 5.6 (4.5 to 7.0).
What type of fluid is 0.9 sodium chloride?
0.9% Normal Saline (NS, 0.9NaCl, or NSS) Normal saline is the chemical name for salt. The generic name is sodium chloride. It is a sterile, nonpyrogenic crystalloid fluid administered via an intravenous solution.
Is 0.45 sodium chloride hypotonic?
Sodium Chloride 0.45% Solution for Infusion is a hypotonic solution, with an approximate osmolarity of 154 mOsm/l.
Is sodium chloride 0.9 hypotonic or hypertonic?
Normal saline (0.9% sodium chloride) contains 308 mOsm/L and is considered isotonic. In contrast, 0.45% sodium chloride (154 mOsm/L) and 0.225% sodium chloride (77 mOsm/L) are hypotonic.
Is sodium chloride 0.9 isotonic hypotonic or hypertonic?
crystalloid isotonicNormal saline solution (0.9% NaCl) or NSS is a crystalloid isotonic IV fluid that contains water, sodium (154 mEq/L), and chloride (154 mEq/L).
What is the difference between normal saline and 0.9 sodium chloride?
Normal saline is 0.9% saline. This means that there is 0.9 G of salt (NaCl) per 100 ml of solution, or 9 G per liter. This solution has 154 mEq of Na per liter. In fact, all the other solutions listed on the previous screen will be compared to normal saline as if it has 150 mEq of Na/L.
What is sodium chloride 0.9 solution used for?
This solution is used to supply water and salt (sodium chloride) to the body. Sodium chloride solution may also be mixed with other medications given by injection into a vein. This solution is usually given by injection into a vein as directed by your doctor.
When is hypotonic saline used?
Hypotonic saline (such as 0.18−0.3% NaCl with dextrose) is commonly used as maintenance fluid in the management of acute infections. In recent years there have been numerous reports of hypotonic saline solutions being associated with adverse outcomes.
Why is 0.9 saline isotonic?
In the water phase a [Na] of 154meq/L contains 154X0. 93=143 meq/l, which is the final concentration when mixed to a liter with the 70ml of solid. 0.9 saline is thus considered “isotonic”.
Is 3% sodium chloride hypotonic?
3% and 5% Sodium Chloride Injection, USP is a sterile, nonpyrogenic, hypertonic solution for fluid and electrolyte replenishment in single dose containers for intravenous administration. The pH may have been adjusted with hydrochloric acid.
What is the hypotonic solution?
Hypotonic solution: A solution that contains fewer dissolved particles (such as salt and other electrolytes) than is found in normal cells and blood. Hypotonic solutions are commonly used to give fluids intravenously to hospitalized patients in order to treat or avoid dehydration.
What does sodium chloride do for the body?
Sodium chloride (NaCl), also known as salt, is an essential compound our body uses to: absorb and transport nutrients. maintain blood pressure. maintain the right balance of fluid.
What is sodium chloride used for?
Sodium chloride is an essential nutrient and is used in healthcare to help prevent patients from becoming dehydrated. It is used as a food preservative and as a seasoning to enhance flavor. Sodium chloride is also used in manufacturing to make plastics and other products. It is also used to de-ice roads and sidewalks.
What is the purpose of using sodium chloride in nasal drops?
It helps add moisture inside the nose to dissolve and soften thick or crusty mucus. In babies and young children with stuffy noses who cannot blow their noses, using this product helps to make the mucus easier to remove with a nasal bulb syringe. This helps relieve stuffiness and makes breathing easier.
What is sodium chloride solution used for?
Sodium chloride 23.4% injection is used to replenish lost water and salt in your body due to certain conditions (eg, hyponatremia or low salt syndrome). It is also used as an additive for total parenteral nutrition (TPN) and carbohydrate-containing IV fluids.
How to understand hypertonic, isotonic, and hypotonic?
To understand hypertonic, isotonic, and hypotonic, you must understand the process of osmosis. With osmosis, just remember LOW to HIGH. Osmosis is the process of molecules moving from a less concentrated solution to a higher concentrated solution by passing through a semipermeable membrane.
Which solution has a lower concentration of solutes?
Hypotonic solutions have a lower concentration of solutes.
Why is isotonic solution used?
Isotonic solution is given to ensure that the cells remain in the extracellular compartment. Goal is to increase the intravascular volume. We want to treat low extracellular fluid so it makes sense that we’d use isotonic solution to keep cells in the extracellular compartment.
What happens to the D5W solution after dextrose is metabolized?
After dextrose is metabolized, the D5W solution becomes hypotonic. (Fluid shift into cells.)
What size IV fluid is used for sterile?
Common IV fluid solution packagings come in different sizes, such as 50mL, 100mL, 250mL, 500mL, and 1000mL. The IV fluid solutions are considered sterile.
What are the symptoms of hypovolemia?
You should know understand and be aware of signs and symptoms of hypovolemia: Poor urine output. Poor skin turgor. Tachycardia. Hypotension. Dehydration. Fluid therapy can be lifesaving and is given when there is a loss of body water. Remember that it can cause a lot of harm when give in the wrong situation.
How to treat IV fluids as a medication?
Treat IV fluids as a medication by observing for allergy response, administering to the right patient, right dosage, right route, right order, and at the right time