Is a pure substance an element or mixture?
2:4019:12Pure Substances and Mixtures, Elements & Compounds ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo pure elements are made of only one type of atom like helium or hydrogen gas those are pureMoreSo pure elements are made of only one type of atom like helium or hydrogen gas those are pure elements molecules are made up of multiple atoms two or more atoms it could be the same type of atom in
Are pure substance and elements the same?
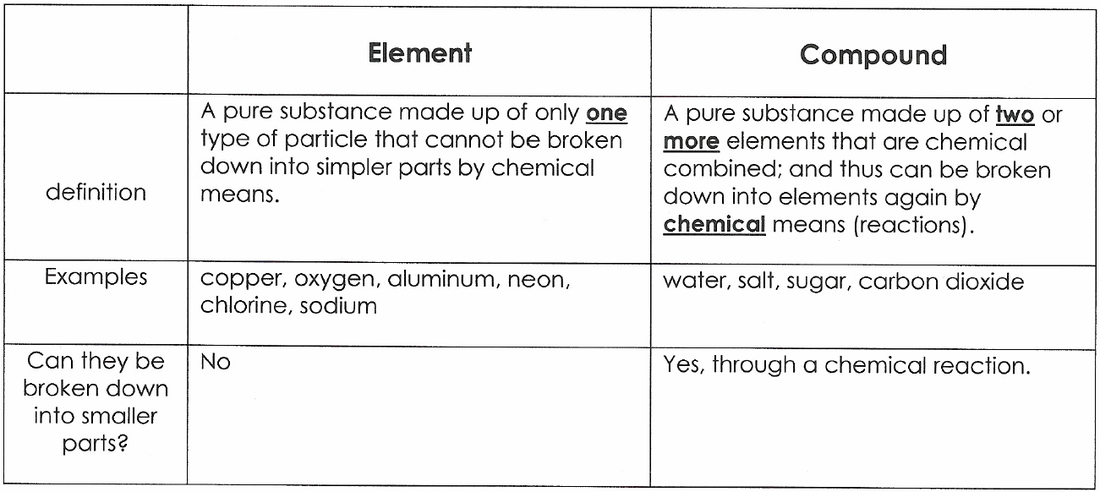
An element is a pure substance that cannot be broken down into different types of substances. There are almost 120 known elements (figure below), each with its own personality. The chemical and physical properties of one element differ from any other.
Is a pure substance a compound?
In a scientific context, “pure” denotes a single type of material. Ostensibly, compounds contain more than one type of material. Yet both compounds and elements are considered pure substances. Pure compounds are created when elements combine permanently, forming one substance.
Why is element not a pure substance?
An element is a pure substance as it cannot be broken down or transformed into a new substance even by using some physical or chemical means. Elements are mostly metals, non-metals or metalloids. Compounds, on the other hand, are also pure substances when two or more elements are combined chemically in a fixed ratio.
What is difference between substance and element?
The difference between substance and element is a substance is a form of matter that takes up space and an element is a pure substance that can NOT be broken down.
Which of these is not an element?
Answer: Among the above-mentioned options, it is (C) Silica that is not an element. In the first option, “Graphite” is an element of carbon. In the same manner “Germanium and Silicon” are also elements but the only remaining option of (C) Silica is not an element.
Is water an element?
Water is a compound because it is made up of water molecules. There is no such thing as water atoms. Water molecules are made of hydrogen and oxygen atoms, in the definite proportion of two hydrogens for one oxygen.
Why is compound called a pure substance?
Compounds are made of only one kind of molecule. The molecule is made up of two or more kinds of atoms. There is no physical change that can separate the compounds into more than one kind of substance. This makes a compound a pure substance.
Why are elements sometimes called pure?
Elements are made of only one kind of atom. The particles can be a single atom or a molecule made of only one kind of atom. There is no physical change that can separate elements into more than one kind of substance. This makes an element a pure substance.
What substance is an element?
An element is a substance that cannot be broken down into a simpler format. They are distinguished by a unique atomic number. The elements are organized by their atomic number in the periodic table, which highlights elements with similar properties.
Which of these pure substances is an element?
Pure SubstancesPure SubstanceElement or Compound?Consists of:Lead (Pb)elementlead atomsOxygen gas (O2)elementoxygen molecules*Water (H2O)compoundwater moleculesAmmonia (NH3)compoundammonia moleculesAug 23, 2019
What classifies a substance as an element?
Elements are chemical substances that are composed of a single kind of atoms and cannot be broken down into simpler forms.
What is pure substance?
A pure substance refers to an element or a compound that has no component of another compound or element. Pure substances are made of only one type of atom or molecule. Hydrogen gas and pure iron are examples of pure substances. Hydrogen consists of hydrogen atoms only while iron consists of only iron atoms. Mixing two pure substances results in ...
What is the degree of purity of a substance?
Degree of Purity of a Substance. The degree of purity of a substance is merely a measure of the extent at which impure substances are present in a substance. It is now apparent that a change in features such as boiling points, however slight, is an indication of the presence of some other substance in that substance.
What is the melting point of water?
Substances that interfere with the purity of a substance are called impurities. Water, for example, has a boiling point of 100°C and a melting point of 0°C. Any change in these values denotes the presence of an impurity. The melting point of a substance should always be similar to its freezing point. When there is a variation, impurities should be ...
How to separate two substances?
To separate the two, scientists use a method known as filtration. Mixtures can either be homogeneous or heterogeneous. The measure used to determine how pure a substance may be called purity. Besides hydrogen and iron, other pure substances include gold, diamonds, sugar, and baking soda.
Is the melting point of a substance always sharp?
The melting point of a substance should always be similar to its freezing point. When there is a variation, impurities should be suspected. The melting and boiling points of pure substances are always sharp.
Is an element a pure substance?
An element is a pure substance and is made of only one type of atom, it cannot be broken down into a simpler substance.
Why are elements and pure substances the same thing?
Elements are made of only one kind of atom. … The molecule is made up of two or more kinds of atoms. There is no physical change that can separate the compounds into more than one kind of substance. This makes a compound a pure substance.
What is the difference between element and pure substance?
Pure substances are substances that are made up of only one kind of particles and has a fixed or constant structure. … An element is a pure substance as it cannot be broken down or transformed into a new substance even by using some physical or chemical means. Elements are mostly metals, non-metals or metalloids.
Do you think all elements are pure substance?
It is something which cannot be divided into parts by physical means, as it’s all made up of the same thing. Pure substances are either elements or compounds. Elements can NOT be separated into other types of matter (physically or chemically). Therefore, elements are pure substances.
What does pure element mean?
A pure element or compound contains only one substance, with no other substances mixed in. Impure materials may be mixtures of elements, mixtures of compounds, or mixtures of elements and compounds. Chemistry.
What are the substance of elements?
A chemical element is a pure substance that consists of one type of atom. Each atom has an atomic number, which represents the number of protons that are in the nucleus of a single atom of that element. The periodic table of elements is ordered by ascending atomic number.
Which of the following is not a pure substance?
Milk is a colloidal solution. It is an emulsion of milk fat in water. Milk comprises fat which is in the form of ester and carbohydrates as sugar. Therefore milk is not a pure substance.
What is pure substance?
A pure substance or chemical substance is a material that has a constant composition (is homogeneous) and has consistent properties throughout the sample. A pure substance participates in a chemical reaction to form predictable products. In chemistry, a pure substance consists ...
What is pure matter?
That is, it is matter that appears uniform in appearance and composition, no matter how small the sample size. Examples of pure substances include iron, steel, and water.
What are some examples of materials that are not pure substances?
Examples of materials that are not pure substances include gravel, your computer, a mixture of salt and sugar, and a tree.
Is a substance a homogeneous substance?
In chemistry, a pure substance has a homogeneous chemical composition.
Is a chemical formula a pure substance?
If you can write a chemical formula for a substance or if it is a pure element, it is a pure substance!
Is baking soda a pure substance?
All elements are pure substances. Sugar, salt, and baking soda are pure substances that are compounds. Examples of pure substances that are crystals include salt, diamond, protein crystals, and copper sulfate crystals. Depending on who you talk to, homogeneous mixtures may be considered examples of pure substances.
What are elements made of?
elements are made up of only one kind of particles. For example: Sulphur (S) is made up of only one particles, that is Sulphur atoms.
What is the definition of an element?
The definition of an element is a specific kind of atom. There is a (semi)fixed number of existing elements, currently at 118. All substances are made by recombining these elements. Which element an atom is is determined by the number of protons, positively charged particles, in the nucleus of the atom. If you have one proton, it’s hydrogen; two and it’s helium. You can’t have an element that’s half hydrogen half helium, it isn’t possible, it’s one or the other. Therefore all elements are by definition pure.
Why do elements combine to form compounds?
So let’s recap: why do elements combine to form compounds? Because most elements aren’t completely stable with the number of valence electrons they have. In most cases, they want to get 8 valence electrons, which they can do by gaining, losing, or sharing electrons with other atoms. When the atoms of two or more elements stick together because of these electron exchanges, a compound is formed.
Which element is the most stable?
The most stable elements are Nobel gases.
Is an element made up of nothing?
Because, by definition, an element is the most basic, chemically active / significant structure. So an element is made up of nothing but itself. An analogy: each letter in the following example is an element. It is isolated when surrounded in both sides by a space. If anything else is adjacent, such as another letter (element) consider that they have combined somehow chemically.
Is water an element?
An element is one of the 92 simplest natural substances that cannot be broken down into a simpler substance. So water for example is not an element because water can be broken down into oxygen and hydrogen. But an element may not be in a pure state. An element is “purely” one type of atom, but a sample of that element may contain impurities.
Is an element a thing?
Elements are by definition just one “thing”.
