What is a Lewis acid?
The aluminum ion is the metal and is a cation with an unfilled valence shell, and it is a Lewis Acid. Water has lone-pair electrons and is an anion, thus it is a Lewis Base. Figure 3: Aluminum ion …
Is AL3+ a Lewis acid or base?
Why is aluminum a Lewis acid? Acidity refers to the strength of an acid (its ability to donate a proton to another molecule). Aluminum chloride (AlCl 3) is a Lewis acid because the aluminum …
Is aluminium a lewic acid or an electrophile?
May 21, 2020 · As an example of a reaction not described by the Bronsted-Lowry definition, Al 3 + in water is a Lewis acid. It reacts with water to form an aqua complex: the Al 3 + accepts an …
Can a proton be a Lewis acid?
In the Lewis theory, an acid is any ion or molecule that can accept a pair of nonbonding valence electrons. In the preceding section, we concluded that Al 3+ ions form bonds to six water …

Is Al 3+ a Lewis acid?
What are considered Lewis acids?
Is alcl3 a Lowry acid?
What is Lewis acid give example?
Why cations are called Lewis acid?
Which is not a Lewis acid?
Is boron trifluoride a Lewis acid?
Why is aluminum a Lewis acid?
Why is AlCl3 considered as Lewis acid?
Are all acids Lewis acids?
Which is the hard acid?
| Acids | Bases | |
|---|---|---|
| hard | Li+, Na+, K+ | H2O, ROH, R2O |
| Be2+, Mg2+, Ca2+, VO2+ | OH−, F−, Cl−, CH3CO2− | |
| Al3+, Sc3+, Cr3+ | CO32− | |
| Ti4+ | PO43− |
What is the Lewis acid?
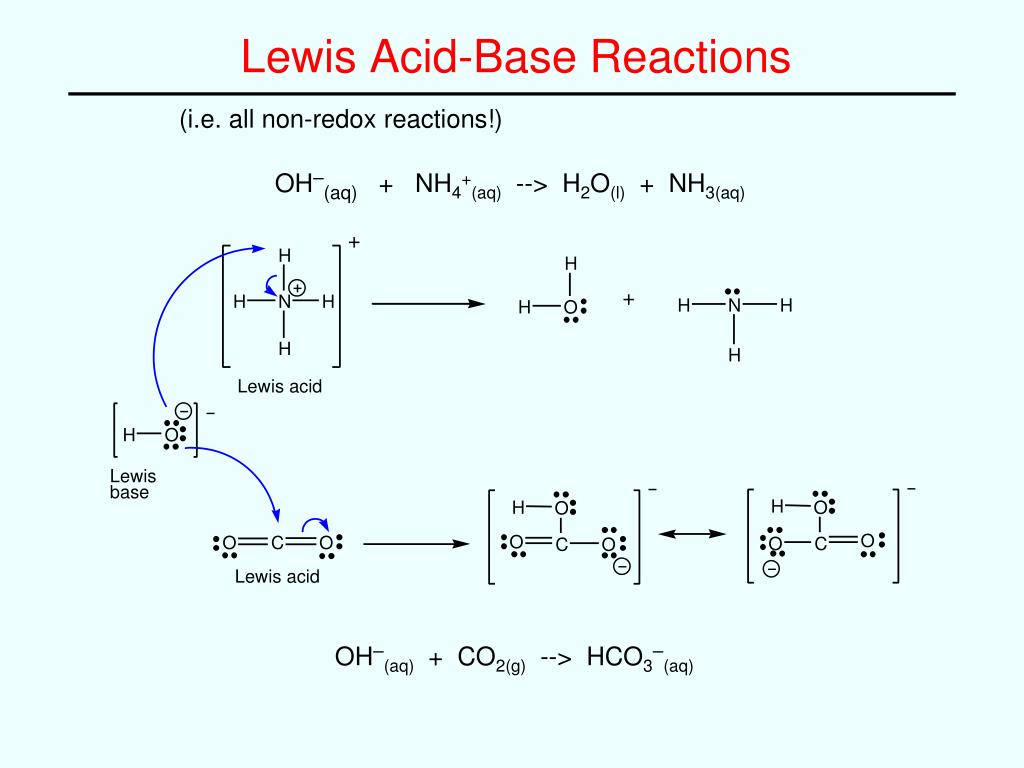
In the Lewis theory of acid-base reactions, bases donate pairs of electrons and acids accept pairs of electrons. A Lewis acid is therefore any substance, such as the H + ion, that can accept a pair of nonbonding electrons. In other words, a Lewis acid is an electron-pair acceptor.
Is Lewis acid a donor or acceptor?
In other words, a Lewis acid is an electron-pair acceptor. A Lewis base is any substance, such as the OH - ion, that can donate a pair of nonbonding electrons. A Lewis base is therefore an electron-pair donor.
What is the Lewis structure of water?
The Lewis structure of water suggests that this molecule has nonbonding pairs of valence electrons and can therefore act as a Lewis base. The electron configuration of the Al 3+ ion suggests that this ion has empty 3 s, 3 p, and 3 d orbitals that can be used to hold pairs of nonbonding electrons donated by neighboring water molecules.
What is Lewis acid?
Lewis proposed a definition for acids and bases that relies on an atom’s or molecule’s ability to accept or donate electron pairs. A Lewis acid is a species that can accept an electron pair, whereas a Lewis base has an electron pair available for donation to a Lewis acid. Complex ions are examples of Lewis acid-base ...
What is the difference between a Lewis acid and a Lewis base?
A Lewis acid is any species (molecule or ion) that can accept a pair of electrons , and a Lewis base is any species (molecule or ion) that can donate a pair of electrons. A Lewis acid-base reaction occurs when a base donates a pair of electrons to an acid. A Lewis acid-base adduct, a compound that contains a coordinate covalent bond between ...
Is HCl a base or acid?
A Brønsted-Lowry acid such as HCl is an acid-base adduct according to the Lewis concept, and proton transfer occurs because a more stable acid-base adduct is formed. Thus, although the definitions of acids and bases in the two theories are quite different, the theories overlap considerably. Many slightly soluble ionic solids dissolve when ...
What are complex ions?
A complex ion consists of a central atom, typically a transition metal cation, surrounded by ions, or molecules called ligands. These ligands can be neutral molecules like H 2 O or NH 3, or ions such as CN – or OH –. Often, the ligands act as Lewis bases, donating a pair of electrons to the central atom.
How do complex ions form?
Complex ions form by sharing electron pairs to form coordinate covalent bonds.

Overview
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct…
Depicting adducts
In many cases, the interaction between the Lewis base and Lewis acid in a complex is indicated by an arrow indicating the Lewis base donating electrons toward the Lewis acid using the notation of a dative bond — for example, Me3B←NH3. Some sources indicate the Lewis base with a pair of dots (the explicit electrons being donated), which allows consistent representation of the transition from the base itself to the complex with the acid:
Lewis acids
Lewis acids are diverse and the term is used loosely. Simplest are those that react directly with the Lewis base, such as boron trihalides and the pentahalides of phosphorus, arsenic, and antimony.
In the same vein, CH3 can be considered to be the Lewis acid in methylation reactions. However, the methyl cation never occurs as a free species in the co…
Lewis bases
A Lewis base is an atomic or molecular species where the highest occupied molecular orbital (HOMO) is highly localized. Typical Lewis bases are conventional amines such as ammonia and alkyl amines. Other common Lewis bases include pyridine and its derivatives. Some of the main classes of Lewis bases are
• amines of the formula NH3−xRx where R = alkyl or aryl. Related to these are pyridine and its deri…
Hard and soft classification
Lewis acids and bases are commonly classified according to their hardness or softness. In this context hard implies small and nonpolarizable and soft indicates larger atoms that are more polarizable.
• typical hard acids: H , alkali/alkaline earth metal cations, boranes, Zn
• typical soft acids: Ag , Mo(0), Ni(0), Pt
Quantifying Lewis acidity
Many methods have been devised to evaluate and predict Lewis acidity. Many are based on spectroscopic signatures such as shifts NMR signals or IR bands e.g. the Gutmann-Beckett method and the Childs method.
The ECW model is a quantitative model that describes and predicts the strength of Lewis acid base interactions, −ΔH. The model assigned E and C parameters to many Lewis acids and bases. Eac…
History
The concept originated with Gilbert N. Lewis who studied chemical bonding. In 1923, Lewis wrote An acid substance is one which can employ an electron lone pair from another molecule in completing the stable group of one of its own atoms. The Brønsted–Lowry acid–base theory was published in the same year. The two theories are distinct but complementary. A Lewis base is also a Brøns…
See also
• Acid
• Base (chemistry)
• Acid–base reaction
• Brønsted–Lowry acid–base theory
• Chiral Lewis acid