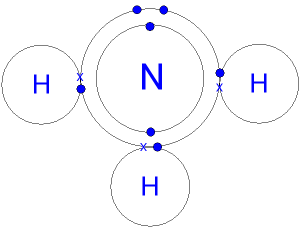
Is ammonia a nucleophile or base?
In other words, nucleophiles are Lewis bases. A nucleophile is either a negative ion or a molecule with a δ⁻ charge somewhere. Ammonia doesn't carry a negative charge. But it has a lone pair of electrons. And nitrogen is more electronegative than hydrogen, so the nitrogen atom has a δ⁻ charge.
Is ammonium ion electrophile or nucleophile?
Thus, the ammonium ion is not an electrophile. People also ask, is nh3 or h20 a better Nucleophile? NH3(Ammonia) has the stronger nucleophilic character than water molecule. Coz the Oxygen in the water molecule doesn't likely to bond with any carbon atom compared to Nitrogen atom.
What is a nucleophile in amines?
A nucleophile is something which is attracted to, and then attacks, a positive or slightly positive part of another molecule or ion. All amines contain an active lone pair of electrons on the very electronegative nitrogen atom. It is these electrons which are attracted to positive parts of other molecules or ions.
Can nitrogen act as a nucleophile?
But it has a lone pair of electrons. And nitrogen is more electronegative than hydrogen, so the nitrogen atom has a δ⁻ charge. So NH₃ can act as a nucleophile and attack the δ⁺ C atom of an alkyl halide. Here's a video on ammonia as a nucleophile.
See more
Is ammonia a nucleophile or electrophile?
nucleophileIn ammonia, nitrogen atom has a lone pair of electrons which works as an electron donor, so ammonia molecule is a nucleophile.
Is ammonia a nucleophile or base?
Ammonia is a nucleophile because it has a lone pair of electrons and a δ⁻ charge on the N atom. A nucleophile is a reactant that provides a pair of electrons to form a new covalent bond.
Is ammonia a good electrophile?
Ammonia can act as both electrophile and nucleophile depending on the need for the reaction. Theoretically, ammonia doesn't have any vacant orbital, therefore, cannot accept any electron. But still, it acts as an electrophile when a strong base attracts the hydrogen of NH3 forming an amide.
Why is ammonium not a nucleophile?
Ammonia as a nucleophile Nucleophiles are either fully negative ions, or else have a strongly - charge somewhere on a molecule. Ammonia obviously doesn't carry a negative charge. However, nitrogen is more electronegative than hydrogen, and so the nitrogen atom carries some degree of negative charge.
Is NH3 a weak or strong nucleophile?
Ammonia still has a lone pair and it is a pretty good nucleophile. We don't need a negative charge on the nitrogen for it to displace a halogen from an alkyl halide. Because nitrogen is a litle less electronegative than oxygen, ammonia is a better nucleophile than water.
Is NH3 a strong base or strong nucleophile?
pKa of NH3 is 38 so it is quite basic. pKa of Ammonium about 9-10, so ammonium is pretty acidic. You're mixing up NH3 with NH2-. The pKa of NH3 is 38 so NH2- is a strong base.
Is ammonia neutral electrophile?
Thus ammonia contains a lone pair of electrons but has no charge. Hence, it is a neutral nucleophile.
Are amines nucleophiles?
Amines act as nucleophiles when the lone pair on the nitrogen atom attacks other organic molecules with areas of partial positive charge.
Which of the following is not a nucleophile?
BF3 is electron deficient compound. It does not have lone pair of electrons to donate. So it is not nucleophilic.
Why NH4 is neither electrophile or nucleophile?
It is neither electrophile nor nucleophile. It is not nucleophile because it cannot donate the other pair of electron on oxygen since Oxygen has positive charge and it will be reluctant with its electronegativity. Positive charge is on oxygen and its octet is filled therefore its not an electrophile.
Is NH4+ not an electrophile?
structure of the ammonium ion (NH4+). In an ammonium ion, nitrogen is bonded to hydrogen atoms and shares electrons with them. This causes all the orbitals to be fully filled and hence, nitrogen does not have space for any additional electrons. Thus, the ammonium ion is not an electrophile.
Which of the following is a nucleophilic?
N) H_(3)`, due to the presence of a lone pair over N, is a nucleophile.
All Answers (7)
Use the Delepine reaction? You may get better results. your epoxide formation is not surprising. Ammonia is not a fantastic nucleophile.
Similar questions and discussions
Can I use triethylamine instead of N,N-Diisopropylethylamine (DIPEA) for acylation of a secondary amine?
What makes a nucleophile a strong reactant?
So, let’s look at what makes strong nucleophiles. There are generally three factors to remember when discussing how nucleophilic a reactant is: 1) Size – Generally, the more linear and/or smaller the nucleophile, the more nucleophilic it will be. This is because it can react at more sites and will not be sterically hindered if it is smaller ...
Can amine bases be nucleophiles?
Here are a couple of good rules to remember: Bases will not be good nucleophiles if they are really bulky or hindered. A variety of amine bases can be bulky and non-nucleophilic. Nucleophiles will not be good bases if they are highly polarizable. I- is the best example of this.
Do nucleophiles have a negative charge?
Think about it for a second….good nucleophiles (as shown above) can have a negative charge and will almost always have a lone pair. Bases accept protons, with a negative charge or lone pair. [gasp] So it makes sense there will be at least some overlap between bases and nucleophiles.
What is a nucleophile?
A nucleophile is something which is attracted to, and then attacks, a positive or slightly positive part of another molecule or ion. All amines contain an active lone pair of electrons on the very electronegative nitrogen atom. It is these electrons which are attracted to positive parts of other molecules or ions.
What reacts with bromoethane to make quaternary ammonium salt?
Making a quaternary ammonium salt. The final stage! The triethylamine reacts with bromoethane to give tetraethylammonium bromide - a quaternary ammonium salt (one in which all four hydrogens have been replaced by alkyl groups). This time there isn't any hydrogen left on the nitrogen to be removed.
What is the function of ethylamine?
The ethylamine removes a hydrogen from the diethylammonium ion to give free diethylamine - a secondary amine. Making tertiary amines and their salts. But it doesn't stop here! The diethylamine also reacts with bromoethane - in the same two stages as before.
Is ethylamine a reversible reaction?
For example if you started with ethylamine and bromoethane, you would get diethylammonium bromide. In the presence of excess ethylamine in the mixture, there is the possibility of a reversible reaction. The ethylamine removes a hydrogen from the diethylammonium ion to give free diethylamine - a secondary amine.