
What are the dangers of ammonium nitrate?
Ammonium Nitratecan affect you when inhaled and by passing through your skin. Contact can irritate and burn the skin and eyes. Inhaling Ammonium Nitratecan irritate the nose, throat and lungs. High levels may cause methemoglobinemiawith headache, Ammonium Nitrateis REACTIVE and a DANGEROUS EXPLOSION HAZARD. Workplace Exposure Limits
What hazard class is ammonium nitrate?
The loss history of AN also indicates potential for unstable reactive hazard properties, uncontrolled decomposition and/or detonation under circumstances that are not fully understood. In the most recent revision of NFPA 400, Hazardous Materials Code, the Technical Committee (TC) classified Ammonium Nitrate as a Class 2 Oxidizer.
What is the environmental impact of ammonium nitrate?
The results of our study showed that environmentally relevant levels of ammonium nitrate can induce mortality and might affect population dynamics of this species in agricultural environments. Publication types Research Support, Non-U.S. Gov't MeSH terms Amphibians / growth & development* Animals Embryonic Development Fertilizers / toxicity*
What is ammonium nitrate, blamed in the Beirut explosion?
When heated, ammonium nitrate includes all of the components required for a fast-burning fire Ammonium nitrate appears to be the cause of the huge explosion that has left large parts of Beirut ruined and at least 100 people dead.
Does ammonium nitrate ignite fire?
Ammonium Nitrate Characteristics It is classified as an oxidizer and will accelerate burning when involved in a fire. Ammonium nitrate itself does not burn, but in contact with other combustible materials it increases fire hazard and supports fire even in the absence of O2.
Will ammonium nitrate explode if burned?
Nitrate of ammonia explosives appears as a powder. Readily explodes if heated. Difficult to explode by shock. Toxic nitrogen oxide fumes may be released during combustion.
What happens if ammonium nitrate is heated?
When ammonium nitrate is heated, it decomposes exothermically into nitrous oxide and water.
What is the hazard of ammonium nitrate?
Irritation and burns Irritation and burns Nose, throat and lung irritation Methemoglobinemia with headache, fatigue and blue color to the skin and lips Cancer - Not tested No information available Remove the person from exposure. Flush eyes with large amounts of water for at least 15 minutes.
What triggers ammonium nitrate to explode?
Over time, the compound absorbs moisture, which can make the beads stick together into a huge rock, says Sella. When such a large quantity of compacted ammonium nitrate is exposed to intense heat — if, say, an accidental fire breaks out — it can trigger an explosion.
What happens if ammonium nitrate gets wet?
Ammonium nitrate forms a mild acid when mixed with water. This acid can cause irritation to the eyes, nose, and skin.
How is ammonium nitrate used as an explosive?
Ammonium nitrate has the chemical formula NH₄NO₃. Produced as small porous pellets, or “prills”, it's one of the world's most widely used fertilisers. It is also the main component in many types of mining explosives, where it's mixed with fuel oil and detonated by an explosive charge.
Does ammonium nitrate expire?
Shelf life – 6 months from the date of production.
Why is ammonium nitrate used as a fertilizer?
Ammonium nitrate is a popular fertilizer since it provides half of the N in the nitrate form and half in the ammonium form. The nitrate form moves readily with soil water to the roots, where it's immediately available for plant uptake.
How do you extinguish ammonium nitrate fire?
Water is the only satisfactory extinguishing material for fires involving Ammonium Nitrate. It is important that the mass be kept cool and that burning materials be promptly extinguished. Large volumes of water should be applied as quickly as possible.
What is the safest way to store ammonium nitrate?
Ammonium nitrate should normally be stored in single storey, dedicated, well-ventilated buildings that are constructed from materials that will not burn, such as concrete, bricks or steel. Clean the store before it is used for ammonium nitrate.
How combustible is ammonium nitrate?
Not combustible but enhances combustion of other substances. Gives off irritating or toxic fumes (or gases) in a fire. Risk of fire and explosion on confinement and exposure to high temperatures or when contaminated with other materials.
At what temperature does ammonia explode?
1. Temperature ranges for which ammonia vapors are explosive in air increase with decreasing am- monia concentration in solutions. For example, ammonia vapors from the 28% solution in air form an explosive atmosphere between 270 and 282 K, whereas it is between 309 and 320 K for a 10% ammonia solution.
How do you extinguish ammonium nitrate fire?
Water is the only satisfactory extinguishing material for fires involving Ammonium Nitrate. It is important that the mass be kept cool and that burning materials be promptly extinguished. Large volumes of water should be applied as quickly as possible.
Can you melt ammonium nitrate?
While ammonium nitrate is noncombustible, it has a melting point near 170oC.
How does ammonium nitrate ignite?
* A large quantity of ammonium nitrate exposed to intense heat can trigger an explosion. Storing the chemical near large fuel tanks, in bulk in large quantities and in a poorly-ventilated facility could cause a massive blast. The larger the quantity, the more risk it will detonate.
What is the name of the salt that is produced by the reaction of ammonia and nitric acid?
Ammonium nitrate is an odorless, colorless or white, crystal salt produced by the reaction of ammonia and nitric acid. How is Ammonium Nitrate used?
How to stop toxic vapors?
If there has been a release of toxic vapors follow these steps: Go indoors immediately and close all doors, windows, and openings. Turn on your radio or television to a local news station. (Be prepared to wait a few minutes for news.) Follow any special instructions given over the radio or television, or by someone in authority.
How to prevent severe inhalation?
For severe inhalation: Use proper respiratory protection to evacuate affected individuals to a safe area as soon as possible. Loosen tight clothing around the neck and waist. Administer oxygen if breathing is difficult. Perform artificial respiration if not breathing.
Can ammonium nitrate cause diarrhea?
When swallowed in high concentrations, ammonium nitrate may cause headache, dizziness, abdominal pain, vomiting, bloody diarrhea, weakness, a tingling sensation, heart and circulation irregularities, convulsions, collapse, and suffocation. Ammonium nitrate forms a mild acid when mixed with water. This acid can cause irritation to ...
Is ammonium nitrate harmful?
Under normal handling conditions, ammonium nitrate is not harmful. However, inhalation of high concentrations of ammonium nitrate dust can cause respiratory tract irritation. Symptoms may include: coughing, sore throat, shortness of breath, or even suffocation.
Can ammonium nitrate explode?
Only under extreme conditions of heat and pressure in a confined space will ammonium nitrate explode. Should such an incident occur, there may be a visible cloud of ammonia, carbon dioxide and nitrogen oxides.
What is ammonium nitrate?
CAMEO Chemicals. Ammonium Nitrate Emulsion, Suspension, or Gel is ammonium nitrate suspended in a liquid. The material itself does not readily burn but will readily do so if contaminated by combustible material.
How much ammonium nitrate is in a solid?
Approximately 60 percent of the ammonium nitrate produced in the U. S. is sold as a solid product. To produce a solid product, the ammonium nitrate solution is concentrated in an evaporator or concentrator. The resulting "melt" contains about 95 to 99.8 percent ammonium nitrate at approximately 149 °C (300 °F).
What is the Ammonium Nitrate Security Program?
Ammonium Nitrate Security Program; Proposed Rule: This proposed rule would implement anti-terrorism measures to better secure the homeland. The Department of Homeland Security would regulate the sale and transfer of ammonium nitrate pursuant to section 563 of the Fiscal Year 2008 Department of Homeland Security Appropriations Act with the purpose of preventing the use of ammonium nitrate in an act of terrorism. This proposed rule seeks comment on both proposed text for such a regulation and on several practical and legal issues integral to the development of an Ammonium Nitrate Security Program.
How to prevent ammonium nitrate explosion?
Actions that may help to prevent explosions include: (1) Avoid heating ammonium nitrate in a confined space (e.g., processes involving ammonium nitrate should be designed to avoid this possibility). (2) Avoid localized heating of ammonium nitrate, potentially leading to development of high temperature areas. (3) Ensure that ammonium nitrate is not exposed to strong shock waves from explosives. (4) Avoid contamination of ammonium nitrate with combustible materials or organic substances such as oils and waxes. (5) Avoid contamination of ammonium nitrate with inorganic materials that may contribute to its sensitivity to explosion, including chlorides and some metals, such as chromium, copper, cobalt, and nickel. (6) Maintain the pH of ammonium nitrate solutions within the safe operating range of the process. In particular, avoid low pH (acidic) conditions.
How is ammonium nitrate produced?
Ammonium nitrate (NH4NO3) is produced by neutralizing nitric acid (HNO3) with ammonia (NH3) . ... All ammonium nitrate plants produce an aqueous ammonium nitrate solution through the reaction of ammonia and nitric acid in a neutralizer.
How many people are exposed to ammonium nitrate?
According to the 2006 TSCA Inventory Update Reporting data, the number of persons reasonably likely to be exposed in the industrial manufacturing, processing, and use of ammonium nitrate is 1000 or greater; the data may be greatly underestimated (1).
What is UN 0222?
UN 0222; Ammonium nitrate, with more than 0.2 percent combustible substances, including any organic substance calculated as carbon, to the exclusion of any other added substance.
Where is ammonium nitrate found?
Ammonium nitrate is found as a natural mineral in the driest regions of the Atacama Desert in Chile, often as a crust on the ground or in conjunction with other nitrate, chlorate and halide minerals. Today almost 100% of the chemical used is synthetically produced.
How fast does ammonium nitrate explode?
If ammonium nitrate explodes, it does so as a deflagration, a rapid auto combustion that does so at a subsonic speed of less than 1,250 feet per second.
What is the critical behavior of fertilizer grade ammonium nitrate?
A critical behavior of fertilizer-grade ammonium nitrate — a strong oxidizer in concentrations above 33.5% — is that it can explode if it becomes contaminated with organic materials such as wood, paper or cardboard.
Is a product flammable at high temperature?
Flammability of the Product: May be combustible at high temperature.
Is static discharge a risk of explosion?
Risks of explosion of the product in presence of static discharge: Not available.
Is ammonium nitrate a good fertilizer?
The good: Ammonium nitrate is commonly used in agriculture as a high-nitrogen fertilizer because it is more stable and does not lose nitrogen to the atmosphere compared with its cousin urea.
What would have made ammonium nitrate more likely to detonate?
Even a small amount of contamination would have made the ammonium nitrate more likely to detonate. Though Lebanese authorities have said ammonium nitrate was the culprit in the blast, Lewis notes that in many cases commercial explosives are referred to as just this compound even though they contain additives.
When was ammonium nitrate first used in explosives?
Add enough of the oxidizer to a fire, and it will trigger an explosion. Though ammonium nitrate was first synthesized in 1659 by German chemist Johann Rudolf Glauber, it wasn’t used in explosives until World War I, when weapon makers mixed it with TNT (aka dynamite) to make cheaper bombs.
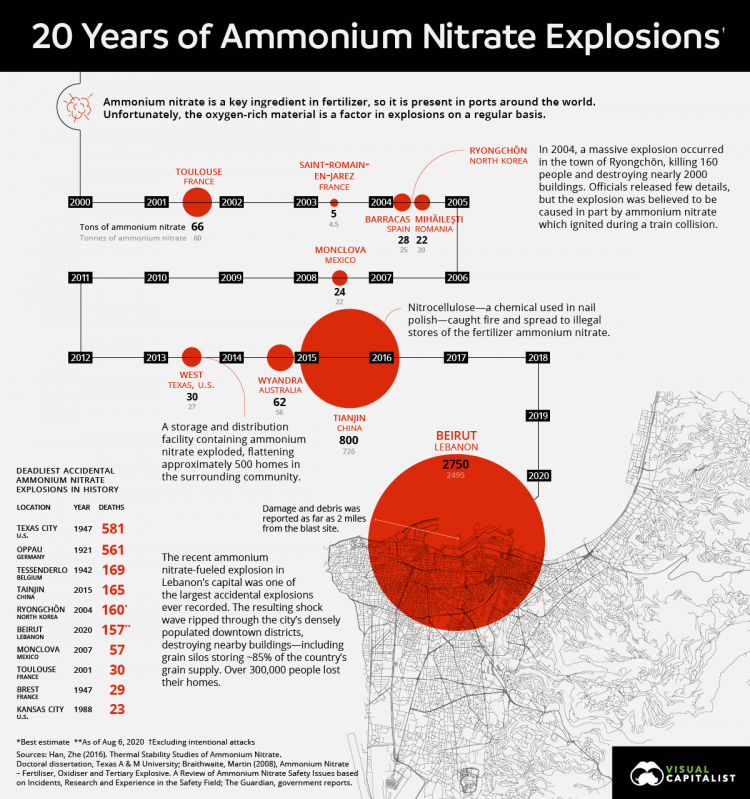
How much ammonium nitrate was used in Beirut?
Blast calculations suggest that at sea level, an explosion like Beirut’s would require about 1,000 tons of ammonium nitrate. Given that these back-of-the-envelope calculations come with a wide margin of error, the estimate is consistent with the 2,750 tons that Lebanese officials say was stored by the port. Or, this discrepancy might mean that the material inside the warehouse was not pure ammonium nitrate, and some other compound was involved with the blast.
What was the explosive that caused the Beirut blast?
The deadly history of ammonium nitrate, the explosive linked to the Beirut blast. A deep-dive into the chemistry and legacy of the compound offers clues into what sparked Lebanon’s catastrophe. A large explosion rocked the Lebanese capital Beirut on August 4. The blast, which rattled entire buildings and broke glass, was felt across the city.
Was ammonium nitrate in Beirut?
A more likely explanation is that the ammonium nitrate in Beirut was not alone inside the warehouse. Given the reported amount, Oxley hypothesizes that a vehicle must have been used to carry the compound inside, and that could have contaminated it with oil or gasoline.
Does ammonium nitrate degrade with time?
But David Chavez, an explosives scientist at the Los Alamos National Laboratory in New Mexico, doubts that scenario. “Ammonium nitrate does not degrade with time under normal storage conditions,” he says. A more likely explanation is that the ammonium nitrate in Beirut was not alone inside the warehouse.
Is ammonium nitrate explosive?
Compared to most combustible materials, ammonium nitrate itself is not exceptionally explosive. But the compound can contribute to explosions because it belongs to a chemical class known as oxidizers.
What temperature does ammonium nitrate decompose?
Ammonium nitrate decomposes in temperatures above 210 °C (410 °F). Pure AN is stable and will stop decomposing once the heat source is removed, but when catalysts are present, the reaction can become self-sustaining (known as self-sustaining decomposition, or SSD).
How many people died in the explosion of ammonium nitrate?
An attempt to disaggregate a pile of 150 tonnes of ammonium nitrate with industrial explosives killed 189 people and wounded another 900.
How many pounds of ammonium nitrate were exploded in 1916?
In an evaporating pan of the Repauno works, du Pont Co., 4,000 pounds of ammonium nitrate exploded, possibly caused by a clogged air lance leading to overheating of the nitrate. 1 man was killed and 12 were injured. United Kingdom. Faversham, Kent. April 2, 1916.
How much ammonium nitrate was consumed in the Repauno disaster?
The column AN states the amount of ammonium nitrate consumed in the disaster in metric tonnes. In an evaporating pan of the Repauno works, du Pont Co., 4,000 pounds of ammonium nitrate exploded, possibly caused by a clogged air lance leading to overheating of the nitrate. 1 man was killed and 12 were injured.
What are the two types of explosions?
There are two major classes of incidents resulting in explosions: 1 In the first case, the explosion happens by the mechanism of shock to detonation transition. The initiation happens by an explosive charge going off in the mass, by the detonation of a shell thrown into the mass, or by detonation of an explosive mixture in contact with the mass. The examples are Kriewald, Morgan, Oppau, Tessenderlo, and Traskwood. 2 In the second case, the explosion results from a fire that spreads into the ammonium nitrate (AN) itself ( Texas City, Brest, Tianjin) or to a mixture of an ammonium nitrate with a combustible material during the fire. The fire must be confined at least to a degree for successful transition from a fire to an explosion (a phenomenon known as "deflagration to detonation transition", or DDT ). Pure, compact AN is stable and very difficult to initiate. However, there are numerous cases when even impure AN did not explode in a fire.
Why is Ammonium Nitrate so explosive?
Ammonium nitrate isn’t an explosive itself; it accelerates the combustion of other materials by providing high volumes of oxygen. This acceleration of combustion is what causes the explosions when ammonium nitrate is mixed with certain chemicals and reactions.
What class of oxidizers are nitrates?
Nitrates belong in a class of oxidizers, react with other elements and compounds to provide oxygen. These oxidizers are very useful and widely used in industries. However, they can form explosive mixture with flammable, organic, or easily oxidized materials. Nitrates also explode by itself when the temperature of the reaction mixture gets very high.
Is nitrogen gas flammable or explosive?
What if you turned your car engine, and the entire world explodes? That is what would have happened if nitrogen gas ever became explosive. Nitrogen makes up about 78 percent of the atmosphere leaving roughly 22 percent oxygen and a few other gases.
Why is Nitrogen Triiodide explosive?
Nitrogen triiodide is a highly explosive chemical that can easily be made from two ingredients, ammonia, and concentrated iodine. As some may have guessed from its name, Nitrogen triiodide is made up of one atom of nitrogen and three atoms of iodine.
What is the difference between Liquid Nitrogen and Nitrogen gas?
There are significant differences between liquid nitrogen and Nitrogen gas. Nitrogen gas is a naturally available substance, but liquid nitrogen does not form naturally and is artificial. Liquid nitrogen is mainly used to preserve items cryogenically.
What happens when nitrogen vaporizes?
When liquid nitrogen vaporizes into cold nitrogen gas, it expands to about seven hundred times in volume. Due to its properties of being colorless and odorless, it is hard to detect. In a confined space, the vaporization of nitrogen can cause oxygen to drop to dangerous levels. This can cause unconsciousness and other asphyxiation hazards.
What is nitrogen gas used for?
In contrast, nitrogen gas is used in many cases, such as chemicals, nylon, dyes, and fertilizers.
Can Fertilizers Cause Cancer?
Fertilizers commonly used at home for lawn and garden care do not tend to be carcinogenic.
Is Fertilizer Flammable?
The vast majority of fertilizers are not flammable and are, in fact, quite chemically stable and not particularly easy to burn.
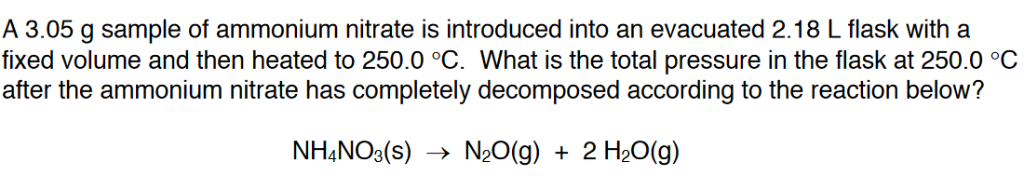
Overview
Safety, handling, and storage
Numerous safety guidelines are available for storing and handling ammonium nitrate. Health and safety data are shown on the safety data sheets available from suppliers and from various governments.
Pure ammonium nitrate does not burn, but as a strong oxidizer, it supports and accelerates the combustion of organic (and some inorganic) material. It should not be stored near combustible …
Occurrence
Ammonium nitrate is found as the natural mineral gwihabaite (formerly known as nitrammite) – the ammonium analogue of saltpetre (mineralogical name: niter) – in the driest regions of the Atacama Desert in Chile, often as a crust on the ground or in conjunction with other nitrate, iodate, and halide minerals. Ammonium nitrate was mined there until the Haber–Bosch process made it possible to synthesize nitrates from atmospheric nitrogen, thus rendering nitrate mining obsolete.
Production, reactions and crystalline phases
The industrial production of ammonium nitrate entails the acid-base reaction of ammonia with nitric acid:
HNO3 + NH3 → NH4NO3
Ammonia is used in its anhydrous form (a gas) and the nitric acid is concentrated. The reaction is violent owing to its highly exothermic nature. After the solution is formed, typically at about 83% …
Applications
Ammonium nitrate is an important fertilizer with NPK rating 34-0-0 (34% nitrogen). It is less concentrated than urea (46-0-0), giving ammonium nitrate a slight transportation disadvantage. Ammonium nitrate's advantage over urea is that it is more stable and does not rapidly lose nitrogen to the atmosphere.
Ammonium nitrate readily forms explosive mixtures with varying properties when combined wit…
Health hazards
Ammonium nitrate is not hazardous to health and is usually used in fertilizer products.
Ammonium nitrate has an LD50 of 2217 mg/kg, which for comparison is about two-thirds that of table salt.
Disasters
Ammonium nitrate decomposes, non-explosively, into the gases nitrous oxide and water vapor when heated. However, it can be induced to decompose explosively by detonation. Large stockpiles of the material can also be a major fire risk due to their supporting oxidation, a situation which can easily escalate to detonation. Explosions are not uncommon: relatively minor incidents occur most years, and several large and devastating explosions have also occurred. Examples include …
See also
• Resource recovery