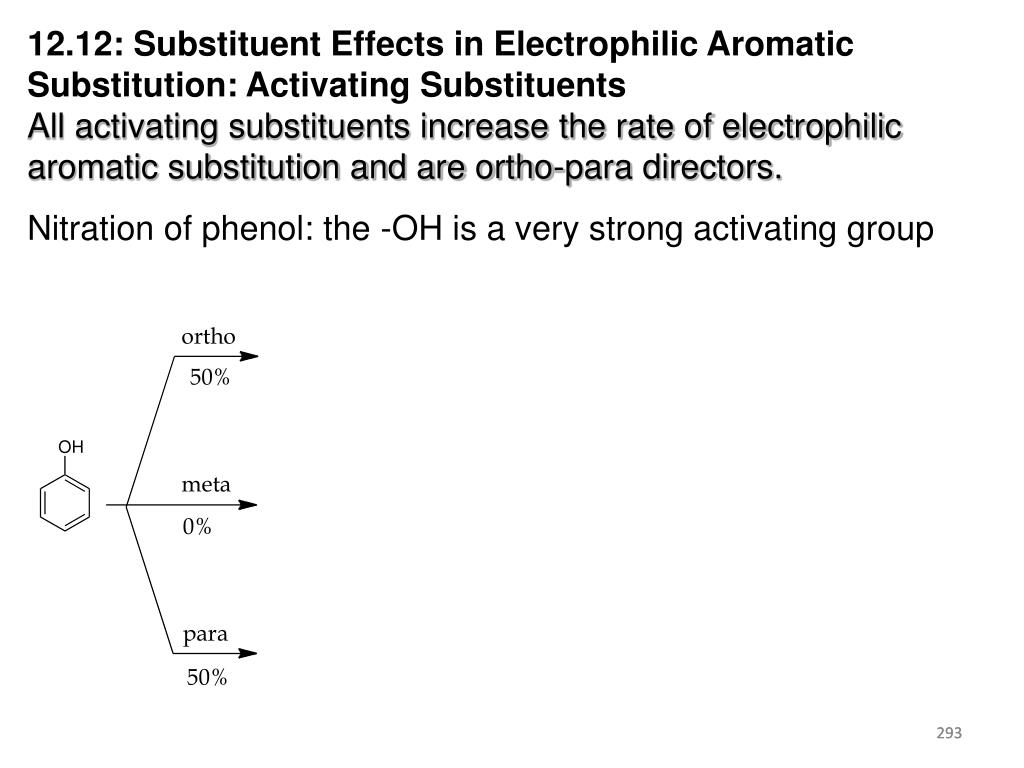
If the substrate is a very reactive benzene derivative, such as anisole, carboxylic esters or acids may be the source of the acylating electrophile.... A common characteristic of the halogenation, nitration, sulfonation and acylation reactions is that they introduce a deactivating substituent on the benzene ring. Are you pack name of anisole?
Full Answer
Which group is the activating group?
What are the effects of an electrophilic aromatic substitution reaction?
Why is phenolate reaction so rapid?
Is a deactivating group a resonance donor?
See 1 more
About this website

Is anisole electron donating or withdrawing?
Anisole, having an ether substituent, has hyperconjugation between the oxygen's filled π orbital electrons and the methyl's C−H σ -bonding electron pair as a opposing effect to the electron donating behavior of −OR , which weakens the electron-donating capacity of the substituent, giving anisole a less strongly- ...
Is phenol or anisole more activating?
Anisole is less reactive than phenol towards electrophilic substitution reactions.
How do you know if it is activating or deactivating?
If a substituent increases the rate of reaction relative to H it is called activating. If it decreases the rate relative to H it is called deactivating.
Is och3 activating or deactivating?
Methoxy group (och3) is an electron-withdrawing group and hence is it is a deactivator.
How do you distinguish between phenol and anisole?
The test tube which forms a violet color immediately is phenol, whereas the test tube in which no coloration is observed is anisole. The principle behind the mechanism is that phenol reacts with ferric chloride, whereas anisole doesn't react with it.
Which is more reactive anisole or benzene?
The order of decreasing reactivity towards electrophilic reagent is d>b>a>c or Anisole > toluene > benzene > chlroro benzene.
Why is COOH ring deactivating?
Oxygen due to electronegativity, it will take the pi bonds making carbon electropositive. This will pull the electrons further attached between the carbons and due to this the positive charge on the arenium ion is further increased rendering it less stable. In this way, carboxylic acid acts as deactivating group.
Why is COOH a deactivating group?
Hence, it acts as a better acid than the equivalent alcohol. For example – Electrophilic substitution does not take place readily for benzene. The incoming individual will join the carboxyl group at Meta position rather than at ortho and para position. Hence, the carboxyl group acts as a deactivating group.
What makes a group activating?
Atoms or groups that make the benzene molecule more reactive by increasing the ring's electron density are called activating groups. Activating groups serve as ortho‐para directors when they are attached to a benzene ring, meaning that they direct an incoming electrophile to the ortho or para positions.
Is OCH3 donating or withdrawing?
b) -OCH3 (methoxy group) The methoxy group is electron withdrawing by the inductive effect of the oxygen atom, since the electronegativity of oxygen is 2.6. This is reflected in the positive value for σm.
Is OCH3 a activating group?
Due to increase in electron density, the ring becomes more activated than unsubstitued benzene towards electrophilic substitution reaction. Thus `-OCH_3` is an activating group.
Is OCH3 a withdrawing or donating group?
Yes, OCH3 which belongs to the is the electron-withdrawing group (methoxy group). Here, the oxygen (in OCH3) is more electronegative than carbon due to which it will show -I effect which is electron-withdrawing.
Why is phenol a strong activator?
The hydroxy group in a phenol molecule exhibits a strong activating effect on the benzene ring because it provides a ready source of electron density for the ring. This directing influence is so strong that you can often accomplish substitutions on phenols without the use of a catalyst.
Is anisole more polar than phenol?
As for phenol, it contains a hydroxyl group which means that it can exhibit hydrogen bonding which is far stronger than the regular dipole-dipole interaction. As such, phenol is more polar than anisole.
Which is more activating OH or OCH3?
+R effect of OCH3 is more than that of OH group.
Which is more reactive anisole or toluene?
So, tentative order of reactivity in electrophilic substitution: Nitrobenzene, acetophenone, benzene, toluene, anisole; i.e. anisole is the most reactive species under these conditions.
Directing Group Influence - CliffsNotes
Substituents already attached to benzene exert an influence on additional atoms or groups attempting to bond to the benzene ring via electrophilic aromatic substitution reactions.
What is the perfect list of electron withdrawing (-I) and electron ...
Answer: Inductive effect (I effect): displacement of sigma electrons along a saturated carbon chain when an electron donating (+I effect) or electron withdrawing (-I effect) group is attached at the end of the carbon chain. * Decreasing order of -I effect : R3N- > -NO2 > -SO2R > -CN > -COOH> -F...
Identifying Electron-Donating Groups - Organic Chemistry - Varsity Tutors
Explanation: . This question tests your knowledge about electron donation, as well as acidity. The question asks you to identify the species with the highest pK a, which means you need to look for the R group that will be the most donating.Electron donation will destabilize the conjugate base anion, localized partially on the oxygen of the hydroxyl moiety; the result is a less acidic acid, and ...
Ch12 : Substituent Effects - Faculty of Science
These effects are a combination of RESONANCE and INDUCTIVE effects (see next page) The effects are also important in other reactions and properties (e.g. acidity of the substituted benzoic acids).Here are some general pointers for recognising the substituent effects: . The H atom is the standard and is regarded as having no effect.; Activating groups increase the rate
Is anisole an acid or a base?
Ethers, such as Anisole can act as bases. They form salts with strong acids and addition complexes with Lewis acids.
How Anisoles are formed?
Anisole can be prepared from phenol or its salts by the use of the following methylating agents: methyl chloride; 1 sodium methyl sulfate; 2 methyl alcohol in the presence of thorium oxide; 3 methyl alcohol and β-naphthalenesulfonic acid 4 or potassium hydrogen sulfate 5 or boron fluoride; 6 dimethyl sulfate; 7 and methyl ...
Is anisole toxic?
Anisole fumes are toxic. Prolonged inhalation may lead to poisoning. The falling drop apparatus using anisole nmst be operated under a fume hood. Extreme caution is recommended in any nse of this compound.
Is anisole flammable?
FIRE HAZARDS * Anisole is a COMBUSTIBLE LIQUID. * Use dry chemical, CO2, water spray, or foam extinguishers.
Is anisole toxic?
Anisole fumes are toxic. Prolonged inhalation may lead to poisoning. The falling drop apparatus using anisole nmst be operated under a fume hood. Extreme caution is recommended in any nse of this compound.
Is anisole flammable?
FIRE HAZARDS * Anisole is a COMBUSTIBLE LIQUID. * Use dry chemical, CO2, water spray, or foam extinguishers.
Making anisole
Anisole is a starting raw material to be chemically transformed by Pharmaceuticals Industries (technical grade). Its appearance is a clear liquid. Anisole presents a wide range of applications: solvent for chemical reactions, synthesis intermediates and solvent for electronics.
This problem has been solved!
1. Based on the mechanism discussed in class, explain briefly whether anisole is activating or deactivating.
Expert Answer
Hello there, 1) Anisole is activating because of OCH3 group. Oxygen lone pair activates the aromatic ring at ortho and para position by increasing electron density on respective … View the full answer
Which group is the activating group?
The activating groups are mostly resonance donors (+M). Although many of these groups are also inductively withdrawing (–I), which is a deactivating effect, the resonance (or mesomeric) effect is almost always stronger, with the exception of Cl, Br, and I.
What are the effects of an electrophilic aromatic substitution reaction?
In an electrophilic aromatic substitution reaction, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. An electron donating group ( EDG) or electron releasing group ( ERG, Z in structural formulas) is an atom or functional group that donates some of its electron density into a conjugated π system via resonance (mesomerism) or inductive effects (or induction)—called +M or +I effects, respectively—thus making the π system more nucleophilic. As a result of these electronic effects, an aromatic ring to which such a group is attached is more likely to participate in electrophilic substitution reaction. EDGs are therefore often known as activating groups, though steric effects can interfere with the reaction.
Why is phenolate reaction so rapid?
Phenol is an ortho/para director, but in a presence of base, the reaction is more rapid. It is due to the higher reactivity of phenolate anion. The negative oxygen was 'forced' to give electron density to the carbons (because it has a negative charge, it has an extra +I effect). Even when cold and with neutral (and relatively weak) electrophiles, the reaction still occurs rapidly.
Is a deactivating group a resonance donor?
Halogen substituents are an exception: they are resonance donors (+M). With the exception of the halides, they are meta directing groups.
Which group is the activating group?
The activating groups are mostly resonance donors (+M). Although many of these groups are also inductively withdrawing (–I), which is a deactivating effect, the resonance (or mesomeric) effect is almost always stronger, with the exception of Cl, Br, and I.
What are the effects of an electrophilic aromatic substitution reaction?
In an electrophilic aromatic substitution reaction, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. An electron donating group ( EDG) or electron releasing group ( ERG, Z in structural formulas) is an atom or functional group that donates some of its electron density into a conjugated π system via resonance (mesomerism) or inductive effects (or induction)—called +M or +I effects, respectively—thus making the π system more nucleophilic. As a result of these electronic effects, an aromatic ring to which such a group is attached is more likely to participate in electrophilic substitution reaction. EDGs are therefore often known as activating groups, though steric effects can interfere with the reaction.
Why is phenolate reaction so rapid?
Phenol is an ortho/para director, but in a presence of base, the reaction is more rapid. It is due to the higher reactivity of phenolate anion. The negative oxygen was 'forced' to give electron density to the carbons (because it has a negative charge, it has an extra +I effect). Even when cold and with neutral (and relatively weak) electrophiles, the reaction still occurs rapidly.
Is a deactivating group a resonance donor?
Halogen substituents are an exception: they are resonance donors (+M). With the exception of the halides, they are meta directing groups.