Can Avastin be used for breast cancer?
Avastin was approved for metastatic breast cancer in 2008 under the accelerated approval program. Under the accelerated approval program, a drug may be approved based on results of clinical data that suggest the drug has an important clinical benefit.
How long is Avastin treatment for breast cancer?
You usually have bevacizumab (Avastin) every 2 to 3 weeks. Treatment usually continues for as long as it controls your cancer.
Why was Avastin taken off the market?
On November 18, 2011, the US Food and Drug Administration (US FDA) announced that breast cancer indication for Avastin (bevacizumab) had been withdrawn after concluding that the drug has not been shown to be safe and effective for the treatment of breast cancer.
Which drug is not approved for the treatment of breast cancer?
On September 8, 2020, the U.S. Food and Drug Administration (FDA) announced it did not approve atezolizumab (Tecentriq®) in combination with paclitaxel for treatment of breast cancer after a clinical trial studying the use of atezolizumab and paclitaxel in patients with previously untreated inoperable locally advanced ...
What is the success rate of Avastin?
Results : Of 133 patients, 106 (80%) achieved treatment stability on Avastin.
What kind of cancer is Avastin used for?
Avastin, in combination with carboplatin and paclitaxel, followed by Avastin alone, is used for the treatment of patients with advanced (Stage III or IV) epithelial ovarian, fallopian tube, or primary peritoneal cancer following initial surgery.
How long do people live on Avastin?
In one study, 637 patients received standard chemotherapy plus radiation, and half also received Avastin. Both groups lived about 16 months, and those on Avastin had more side effects — mostly low blood counts, blood clots and high blood pressure.
Is Avastin still used?
The medicine itself is not being removed from the market and doctors can choose to use Avastin to treat metastatic breast cancer whether or not that particular use is officially approved by the FDA. Avastin is also approved by the FDA to treat advanced cancers of the lung, colon, and rectum.
How many years can you take Avastin?
And you keep taking Avastin as long as your disease is controlled and your side effects are manageable, up to 22 cycles. By continuing to take Avastin, you may be able to continue to control your cancer.
What is the hardest form of breast cancer to treat?
What is triple-negative breast cancer? Triple-negative breast cancer is that which tests negative for three receptors: estrogen, progesterone, and human epidermal growth factor receptor 2 (HER2). It is also the least common form of breast cancer and the hardest to treat.
What is the newest treatment for breast cancer?
New drugs, known as cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors — which include palbociclib, ribociclib, and abemaciclib — have transformed the standard of cancer treatment by interrupting cancer cell growth, according to an analysis published in 2021 in JCO Oncology Practice.
What is the new drug for breast cancer?
Adding the immunotherapy drug pembrolizumab (Keytruda) to chemotherapy can help some patients with advanced triple-negative breast cancer live longer than if they received chemotherapy alone, new results from a clinical trial show.
How long can a patient stay on Avastin?
And you keep taking Avastin as long as your disease is controlled and your side effects are manageable, up to 22 cycles. By continuing to take Avastin, you may be able to continue to control your cancer.
What is the average length of treatment for breast cancer?
Each treatment session is followed by a period of recovery. Typically, if you have early-stage breast cancer, you'll undergo chemotherapy treatments for three to six months, but your doctor will adjust the timing to your circumstances. If you have advanced breast cancer, treatment may continue beyond six months.
How long do people live on Avastin?
In one study, 637 patients received standard chemotherapy plus radiation, and half also received Avastin. Both groups lived about 16 months, and those on Avastin had more side effects — mostly low blood counts, blood clots and high blood pressure.
How long do Avastin infusions take?
How long do my Avastin infusions take? You always get the same dose of Avastin. If your Avastin infusions are tolerated, they can take as little as 30 minutes. Your doctor or nurse will monitor you for signs of infusion-related reactions, and may stop Avastin treatment if severe reactions occur.
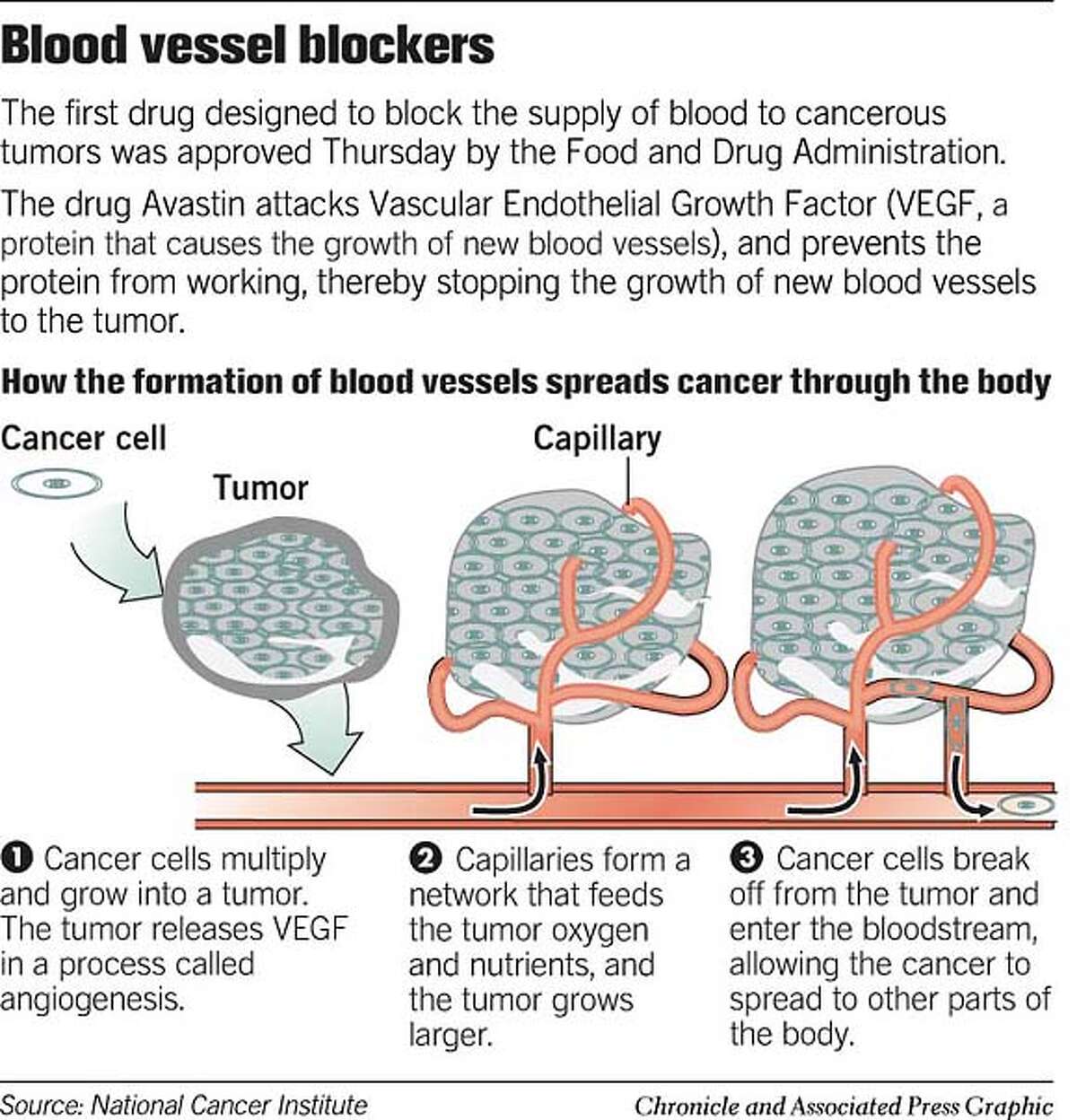
How does Avastin work?
Avastin is an injectable cancer medication that works by blocking a protein that is important for the formation of blood vessels. Since tumors rely on blood vessels to get the nutrients they need to survive, the drug is thought to work by preventing the formation of new blood vessels that feed the tumor . Avastin was first approved in 2004 for treatment of advanced colon cancer and has been approved since for advanced lung (2006), kidney and brain (glioblastoma) cancers (2009). Avastin was approved for metastatic breast cancer in 2008 under the accelerated approval program. Under the accelerated approval program, a drug may be approved based on results of clinical data that suggest the drug has an important clinical benefit. However, additional information is needed to confirm the data.
When was Avastin first approved?
Avastin was first approved in 2004 for treatment of advanced colon cancer and has been approved since for advanced lung (2006), kidney and brain (glioblastoma) cancers (2009). Avastin was approved for metastatic breast cancer in 2008 under the accelerated approval program.
Is Avastin safe for breast cancer?
The FDA has proposed withdrawing Avastin's indication to treat women with metastatic breast cancer. The agency is making this announcement after deciding there is not enough evidence that the drug is safe and effective for this one indication. The announcement will not have any immediate effect on the approval of Avastin to treat metastatic breast ...
Is Genentech requesting a hearing?
FDA has received a submission from Genentech supporting its request for a Notice of Opportunity for a Hearing. The information has been submitted to the docket (FDA-2010-N-0621) and was due to the agency by close of business today. We are at the beginning stages of this process and the agency will review the materials in order to make a determination on whether a public hearing for Avastin in metastatic breast cancer is warranted.
Is Avastin still on the market?
That is, regardless of the final decision on metastatic breast cancer approval, Avastin will remain on the market.
Does Avastin have a label change?
The announcement will not have any immediate effect on the approval of Avastin to treat metastatic breast cancer and there will be no change to product labeling. Since the marketing approval remains in effect, patients with breast cancer will still have access to the drug until a final decision has been made.
Is Avastin approved based on clinical data?
Under the accelerated approval program, a drug may be approved based on results of clinical data that suggest the drug has an important clinical benefit. However, additional information is needed to confirm the data. The FDA has proposed withdrawing Avastin's indication to treat women with metastatic breast cancer.
What is the class of drugs that thwarts tumors?
Avastin belongs to a class of drugs called angiogenesis inhibitors, which thwarts tumors from making new new blood vessels. By preventing the growth of new blood vessels, the drug starves tumors.
Why was breast cancer removed from the FDA?
But in July 2010, an FDA advisory panel voted 12-1 to remove the breast cancer indication from the drug’s label because follow-up studies found no differences in overall survival. These studies also showed that progression-free survival improved by less than three months, and that there was a high rate of side effects.
When was Avastin approved?
In 2008, the FDA approved Avastin as a breast cancer treatment for some women. That approval, which was fast-tracked, was based on preliminary studies that found that the drug increased progression-free survival -- the time in which women's breast cancer did not worsen.
When did the FDA stop avastin?
The FDA started the process of removing Avastin's breast cancer indication in 2010. Genentech, the drug company that makes Avastin, appealed, completed two more studies, and submitted more data to the FDA. But now, the FDA's decision is final.
Is the FDA decision on breast cancer difficult?
"FDA recognizes how hard it is for patients and their families to cope with metastatic breast cancer and how great a need there is for more effective treatments.
Is Avastin still approved for breast cancer?
The cancer drug has lost its FDA approval as a treatment for breast cancer patients. Here's why. By Denise Mann. FDA's Decision. Avastin and Breast Cancer. The FDA has ruled that the cancer drug Avastin is no longer approved for treating advanced breast cancer -- but can still be used for other cancers. In a news release, the FDA stated that ...
Is Avastin still available off label?
But now, the FDA's decision is final . Doctors will still have the option of using Avastin off-label to treat breast cancer, Lillie Shockney, RN, administrator of the Johns Hopkins Breast Center in Baltimore, told WebMD earlier this year.
What is Avastin used for?
Food and Drug Administration (FDA) to be used in combination with Taxol (chemical name: paclitaxel) to treat metastatic, HER2-negative breast cancer that hasn't yet been treated with chemotherapy. Since the beginning of 2011, ...
What is the treatment for metastatic breast cancer?
If you've been diagnosed with metastatic breast cancer, you and your doctor will develop a treatment plan that will likely include chemotherapy and possibly hormonal therapy and/or targeted therapy medicines . No matter which treatments are recommended for you, you may want to talk to your doctor about:
When did the FDA remove Avastin?
Since the beginning of 2011, the FDA has been considering removing the breast cancer indication. On Nov. 18, 2011 FDA Commissioner Margaret Hamburg decided to remove it. When she announced her decision, Hamburg said available research shows that women who get Avastin to treat metastatic breast cancer risk potentially life-threatening side effects ...
What is the oldest estrogen receptor modulator?
Breast Self-Exam. Breast self-exam, or regularly examining your breasts on your own, can be an important way to... Tamoxifen (Brand Names: Nolvadex, Soltamox) Tamoxifen is the oldest and most-prescribed selective estrogen receptor modulator (SERM)....
What is the oldest and most prescribed selective estrogen receptor modulator?
Tamoxifen is the oldest and most-prescribed selective estrogen receptor modulator (SERM)....
Does Genentech use Avastin?
Genentech remains committed to evaluating the use of Avastin to treat metastatic breast cancer. The company plans to conduct new research focusing on identifying the women who are most likely to benefit from Avastin treatment. Stay tuned to Breastcancer.org Research News for information about any results.
Does Medicare pay for Avastin?
Still, the day after the June 2011 FDA expert panel's recommendation was released, a spokesperson for Medicare announced that even if the Avastin breast cancer indication was removed, Medicare would continue to pay for Avastin to treat metastatic breast cancer. Medicare's announcement is important because many insurers follow Medicare's payment policies. Medicare and insurance companies often pay for treatments prescribed by doctors for a condition even if the treatment isn't FDA-approved for that specific use. The NCCN's continued support for Avastin to treat metastatic breast cancer also should help encourage some insurers to continue to pay for it despite the final FDA decision.