...
5. What are carboxylic acids?
| Carbon atoms | 4 |
|---|---|
| Common name | Butyric acid |
| IUPAC name | Butanoic acid |
| Chemical formula | CH 3(CH 2) 2COOH |
| Common location or use | Rancid butter |
What is the chemical formula for butanoic acid?
Butanoic acid also called butyric acid is a carboxylic acid with the chemical formula C4H8O2. Visit BYJU'S to understand the properties, structure and uses of butanoic acid.
What is another name for butyric acid?
Butyric acid (from Ancient Greek: βούτῡρον, meaning "butter"), also known under the systematic name butanoic acid, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates.
What are butyrates and butanoates?
Salts and esters of butyric acid are known as butyrates or butanoates. Butyric acid is found in animal fat and plant oils, bovine milk, breast milk, butter, parmesan cheese, and as a product of anaerobic fermentation (including in the colon and as body odor, and vomit ). Butyric acid has a taste somewhat like butter...
What is butanoic acid used for?
Uses of Butanoic acid – C 4H 8O 2 Used in the manufacture of esters for artificial flavorings, as a food additive, in the manufacture of varnishes, and in decalcifying hides. Used in the manufacture of perfume, flavorings, pharmaceuticals, and disinfectants.

What type of hydrocarbon is butanoic acid?
Butyric acid, also known as butanoic acid or butyrate, belongs to the class of organic compounds known as straight chain fatty acids. These are fatty acids with a straight aliphatic chain....Structure for #
What type of hydrocarbon Is carboxylic acid?
Carboxylic acids are hydrocarbon compounds in which a carboxyl group has substituted one or more of the hydrogen atoms in the hydrocarbon. Methanoic acid (HCOOH), ethanoic acid (CH3COOH), propanoic acid (C2H5COOH), and butanoic acid (C3H7COOH) are the first four carboxyl acids derived from alkanes.
Is butanoic acid a carboxylic acid?
Butyric acid (/ˈbjuːtɪrɪk/; from Ancient Greek: βούτῡρον, meaning "butter"), also known under the systematic name butanoic acid, is a straight-chain alkyl carboxylic acid with the chemical formula CH3CH2CH2CO2H.
Which is an example of a carboxylic acid?
Carboxylic acids feature a carbon atom doubly bonded to an oxygen atom and also joined to an OH group. The four acids illustrated here are formic acid (a), acetic acid (b), propionic acid (c), and butyric acid (d).
Which is an acidic hydrocarbon?
Propyne (CH3C≡CH) has one acidic hydrogen i.e. the terminal hydrogen bond to C via triple bond.
Is carboxylic acid an ester?
What are esters? Esters are derived from carboxylic acids. A carboxylic acid contains the -COOH group, and in an ester the hydrogen in this group is replaced by a hydrocarbon group of some kind.
Is butanoic acid an ester?
butyric acid (CH3CH2CH2CO2H), also called butanoic acid, a fatty acid occurring in the form of esters in animal fats and plant oils. As a glyceride (an ester containing an acid and glycerol), it makes up 3–4 percent of butter; the disagreeable odour of rancid butter is that of hydrolysis of the butyric acid glyceride.
What is the functional group of butanoic acid?
Oxygen basedChemical classGroupExampleAldehydeCarbonylButyraldehyde (Butanal)KetoneCarbonyl2-Butanone (Methyl ethyl ketone)Carboxylic AcidCarboxylButanoic acid (Butyric acid)Acyl halideHaloformylButanoyl bromide (Butyryl bromide)21 more rows•Sep 12, 2020
What is the common name of butanoic acid?
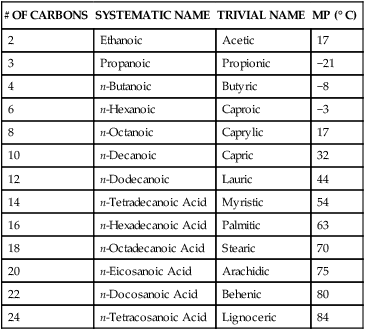
acid butyric acidCarboxylic Acids#CIUPAC NameCommon Name2ethanoic acidacetic acid3propanoic acidpropionic acid4butanoic acidbutyric acid5pentanoic acidvaleric acid2 more rows
What are the types of carboxylic acids?
Carboxylic acids feature a carbon atom doubly bonded to an oxygen atom and also joined to an OH group. The four acids illustrated here are formic acid (a), acetic acid (b), propionic acid (c), and butyric acid (d).
What are the first 5 carboxylic acids?
Carboxylic acids are derivatives of hydrocarbons in which one or more of the hydrogen atoms in the hydrocarbon have been replaced by a carboxyl group. The first four carboxylic acids derived from alkanes are methanoic acid (HCOOH), ethanoic acid (CH3COOH), propanoic acid (C2H5COOH) and butanoic acid (C3H7COOH).
Which functional group is a carboxylic acid?
The carboxyl functional group that characterizes the carboxylic acids is unusual in that it is composed of two functional groups: (1) the carboxyl group and (2) of a hydroxyl group bonded to a carbonyl group. It is often written in condensed form as –CO2H or –COOH.
Which functional group is a carboxylic acid?
The carboxyl functional group that characterizes the carboxylic acids is unusual in that it is composed of two functional groups: (1) the carboxyl group and (2) of a hydroxyl group bonded to a carbonyl group. It is often written in condensed form as –CO2H or –COOH.
Is carboxylic acid saturated or unsaturated?
A fatty acid is a carboxylic acid with a long, aliphatic chain. This chain is either saturated (it has no double bonds) or unsaturated (it contains double bonds). In oil paint, fatty acids are obtained from triglyceride molecules.
How do you identify carboxylic acids?
Prepare a saturated solution of sodium bicarbonate by dissolving sodium bicarbonate in 1ml of water. Add the given organic compound to the saturated solution of sodium bicarbonate solution. Shake the solution well. If there is an evolution of brisk effervescence then it indicates the presence of carboxylic acid.
What Is carboxylic acid in organic chemistry?
carboxylic acid, any of a class of organic compounds in which a carbon (C) atom is bonded to an oxygen (O) atom by a double bond and to a hydroxyl group (―OH) by a single bond.
What is Butanoic acid?
Butanoic acid is an oily colourless liquid with the chemical formula . It is a short chain saturated fatty acid found in the form of esters in animal fats and plant oils. It was discovered by Lieben and Rossi in 1869. It is also called butyric acid which means the acid of butter as it was first discovered in rancid butter. It was prepared by the butyric fermentation of carbohydrates and by the oxidation of n-butyl alcohol.
How is butyric acid made?
Butyric acid is industrially prepared by Butyraldehyde oxidation. Saturation with salts such as calcium chloride can isolate it from the aqueous solutions. When dissolved in hot water, the calcium salt, Ca (C4H7O2)2·H2O, is relatively less soluble.
What is butyrate produced by?
Butyrate is generated by multiple fermentation processes performed by obligatory anaerobic bacteria. Louis Pasteur discovered this fermentation pathway in 1861. Examples of bacteria which produce butyrate include Clostridium butyricum, Clostridium kluyveri and Clostridium pasteurianum
What is the chemical formula for butyric acid?
Butyric acid (from Ancient Greek: βούτῡρον, meaning "butter"), also known under the systematic name butanoic acid, is a straight-chain alkyl carboxylic acid with the chemical formula CH 3 CH 2 CH 2 CO 2 H. It is an oily, colorless liquid with an unpleasant odor. Isobutyric acid (2-methylpropanoic acid) is an isomer. Salts and esters of butyric acid are known as butyrates or butanoates. The acid does not occur widely in nature, but its esters are widespread. It is a common industrial chemical and an important component in the mammalian gut.
What is butyric acid used for?
Uses. Butyric acid is used in the preparation of various butyrate esters. It is used to produce cellulose acetate butyrate (CAB), which is used in a wide variety of tools, paints, and coatings, and is more resistant to degradation than cellulose acetate.
Why is butyrate important?
Butyrate is essential to host immune homeostasis. Although the role and importance of butyrate in the gut is not fully understood, many researchers argue that a depletion of butyrate-producing bacteria in patients with several vasculitic conditions is essential to the pathogenesis of these disorders. A depletion of butyrate in the gut is typically caused by an absence or depletion of butyrate-producing-bacteria (BPB). This depletion in BPB leads to microbial dysbiosis. This is characterized by an overall low biodiversity and a depletion of key butyrate-producing members. Butyrate is an essential microbial metabolite with a vital role as a modulator of proper immune function in the host. It has been shown that children lacking in BPB are more susceptible to allergic disease and Type 1 Diabetes. Butyrate is also reduced in a diet low in fiber which can induce inflammation and have other adverse affects insofar as these short-chain fatty acids activate PPAR-γ.
What is the name of the compound that is a butyrate?
The butyrate or butanoate, ion is C 2 H 5 C O O −, the conjugate base of butyric acid. It is the form found in biological systems at physiological pH. A butyric, or butanoic, compound is a carboxylate salt or ester of butyric acid.
What is the name of the XM ligase that metabolizes butyric acid?
Butyric acid is metabolized by various human XM-ligases (ACSM1, ACSM2B, ASCM3, ACSM4, ACSM5, and ACSM6), also known as butyrate–CoA ligase . The metabolite produced by this reaction is butyryl–CoA, and is produced as follows:
What happens when butter goes rancid?
Occurrence. Triglycerides of butyric acid compose 3–4% of butter. When butter goes rancid, butyric acid is liberated from the glyceride by hydrolysis. It is one of the fatty acid subgroup called short-chain fatty acids. Butyric acid is a typical carboxylic acid that reacts with bases and affects many metals.
When was butyric acid first discovered?
Butyric acid was first observed in impure form in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. However, Chevreul did not publish his early research on butyric acid; instead, he deposited his findings in manuscript form with the secretary of the Academy of Sciences in Paris, France.
What is a carboxylic acid?
Carboxylic acids are members of a homologous series (family) of molecules which have an COOH group attached to a hydrocarbon chain.
How many carbons are in ethanoic acid?
So ethanoic acid has 2 carbons ("eth"), no carbon-carbon double bonds ("an") and a COOH group ("oic acid"). It doesn't matter that you have already counted that carbon in the first part of the name.
What is the reaction between magnesium and hydrogen gas?
Hydrogen gas is produced and the magnesium reacts with the acid to give a colourless solution of magnesium ethanoate.
What does acid smell like?
The only one you are likely to come across is ethanoic acid which smells very powerfully of vinegar.
What is the pH of ethanoic acid?
Ethanoic acid solution typically has a pH in the region 2 - 3 depending on the concentration . That's significantly higher than the pH of hydrochloric acid of the same sort of concentrations.
What is the reaction between carbon dioxide and sodium carbonate?
Carbon dioxide is produced and the sodium carbonate reacts to give a colourless solution of sodium ethanoate.
Does ethanoic acid contain hydrogen?
So ethanoic acid solution (and solutions of the other carboxylic acids) contain hydrogen ions, but not as many as hydrochloric acid of the same concentration. This shows in their reactions.
What are the hydrocarbons in nature?
The chemical hydrocarbon composition varies between age, sex, nest location, and hierarchal position.
What is a hydrocarbon?
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic with only weak odours. Because of their diverse molecular structures, it is difficult to generalize further.
Why are hydrocarbons used in heating?
Hydrocarbons are currently the main source of the world's electric energy and heat sources (such as home heating) because of the energy produced when they are combusted. Often this energy is used directly as heat such as in home heaters, which use either petroleum or natural gas. The hydrocarbon is burnt and the heat is used to heat water, which is then circulated. A similar principle is used to create electrical energy in power plants .
What are the different types of hydrocarbons?
As defined by IUPAC nomenclature of organic chemistry, the classifications for hydrocarbons are: 1 Saturated hydrocarbons are the simplest of the hydrocarbon species. They are composed entirely of single bonds and are saturated with hydrogen. The formula for acyclic saturated hydrocarbons (i.e., alkanes) is C n H 2n+2. The most general form of saturated hydrocarbons is C n H 2n+2 (1-r), where r is the number of rings. Those with exactly one ring are the cycloalkanes. Saturated hydrocarbons are the basis of petroleum fuels and are found as either linear or branched species. Substitution reaction is their characteristics property (like chlorination reaction to form chloroform ). Hydrocarbons with the same molecular formula but different structural formulae are called structural isomers. As given in the example of 3-methylhexane and its higher homologues, branched hydrocarbons can be chiral. Chiral saturated hydrocarbons constitute the side chains of biomolecules such as chlorophyll and tocopherol. 2 Unsaturated hydrocarbons have one or more double or triple bonds between carbon atoms. Those with double bond are called alkenes. Those with one double bond have the formula C n H 2n (assuming non-cyclic structures). Those containing triple bonds are called alkyne. Those with one triple bond have the formula C n H 2n−2. 3 Aromatic hydrocarbons, also known as arenes, are hydrocarbons that have at least one aromatic ring. 10% of total nonmethane organic carbon emission are aromatic hydrocarbons from the exhaust of gasoline-powered vehicles.
Why are hydrocarbons harmful to the environment?
Hydrocarbons are introduced into the environment through their extensive use as fuels and chemicals as well as through leaks or accidental spills during exploration, production, refining, or transport of fossil fuels. Anthropogenic hydrocarbon contamination of soil is a serious global issue due to contaminant persistence and the negative impact on human health.
Where are hydrocarbons found?
Some hydrocarbons also are widespread and abundant in the solar system. Lakes of liquid methane and ethane have been found on Titan, Saturn 's largest moon, confirmed by the Cassini-Huygens Mission. Hydrocarbons are also abundant in nebulae forming polycyclic aromatic hydrocarbon (PAH) compounds.
What are hydrocarbons with the same molecular formula but different structural formulae called?
Hydrocarbons with the same molecular formula but different structural formulae are called structural isomers. As given in the example of 3-methylhexane and its higher homologues, branched hydrocarbons can be chiral. Chiral saturated hydrocarbons constitute the side chains of biomolecules such as chlorophyll and tocopherol.

Overview
Butyric acid (from Ancient Greek: βούτῡρον, meaning "butter"), also known under the systematic name butanoic acid, is a straight-chain alkyl carboxylic acid with the chemical formula CH3CH2CH2CO2H. It is an oily, colorless liquid with an unpleasant odor. Isobutyric acid (2-methylpropanoic acid) is an isomer. Salts and esters of butyric acid are known as butyrates or butanoates. The acid does not occur widely in nature, but its esters are widespread. It is a com…
History
Butyric acid was first observed in impure form in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. However, Chevreul did not publish his early research on butyric acid; instead, he deposited his findings in manuscript form with the secretary of the Academy of Sciences in Paris, France. Henri Braconnot, a French chemist, was also researching the composition of butter and was publishing his findings, and this led to d…
Occurrence
Triglycerides of butyric acid compose 3–4% of butter. When butter goes rancid, butyric acid is liberated from the glyceride by hydrolysis. It is one of the fatty acid subgroup called short-chain fatty acids. Butyric acid is a typical carboxylic acid that reacts with bases and affects many metals. It is found in animal fat and plant oils, bovine milk, breast milk, butter, parmesan cheese, body odor, vomit, and as a product of anaerobic fermentation (including in the colon). It has a taste somewha…
Production
In industry, butyric acid is produced by hydroformylation from propene and syngas, forming butyraldehyde, which is oxidised to the final product.
H2 + CO + CH3CH=CH2 → CH3CH2CH2CHO → butyric acid
It can be separated from aqueous solutions by saturation with salts such as calcium chloride. The calcium salt, Ca(C4H7O2)2·H2O, is less soluble in hot w…
Reactions
Butyric acid reacts as a typical carboxylic acid: it can form amide, ester, anhydride, and chloride derivatives. The latter, butyryl chloride, is commonly used as the intermediate to obtain the others.
Uses
Butyric acid is used in the preparation of various butyrate esters. It is used to produce cellulose acetate butyrate (CAB), which is used in a wide variety of tools, paints, and coatings, and is more resistant to degradation than cellulose acetate. CAB can degrade with exposure to heat and moisture, releasing butyric acid.
Low-molecular-weight esters of butyric acid, such as methyl butyrate, have mostly pleasant aro…
Pharmacology
Butyric acid (pKa 4.82) is fully ionized at physiological pH, so its anion is the material that is mainly relevant in biological systems. It is one of two primary endogenous agonists of human hydroxycarboxylic acid receptor 2 (HCA2, aka GPR109A), a Gi/o-coupled G protein-coupled receptor (GPCR),
Like other short-chain fatty acids (SCFAs), butyrate is an agonist at the free fatty acid receptors FF…
Biochemistry
Butyrate has numerous effects on energy homeostasis and related diseases (diabetes and obesity), inflammation, and immune function (e.g., it has pronounced antimicrobial and anticarcinogenic effects) in humans. These effects occur through its metabolism by mitochondria to generate ATP during fatty acid metabolism or through one or more of its histone-modifying enzyme targets (i.e., the class I histone deacetylases) and G-protein coupled receptor targets (i.e., FFAR2, FFAR3, and