Is CH4 a molecule or formula unit?
NaCl is its formula unit. That formula unit consists of sodium cation and chloride. Methane is a covalent compound. Its formula unit is CH4 (4 as a subscript). That formula unit is a molecule of CH4. When Ionic compounds dissolve in water, the dissociate into ions.
What is the molecular structure of CH4?
What Is the Molecular Geometry of CH4? CH4, commonly known as methane, is a tetrahedral structure with four hydrogen atoms forming around a central carbon atom. Pictorially, this structure resembles a pyramid in shape, with all four corners equidistant from the center.
What is the molar mass of CH4?
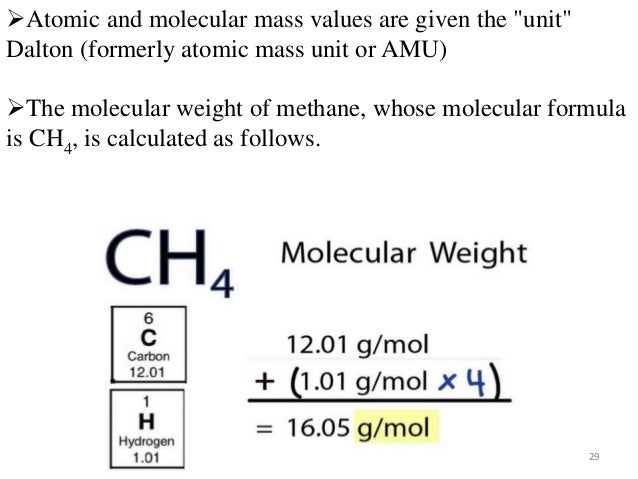
Molar mass of CH4 = 16.04246 g/mol. This compound is also known as Methane. Convert grams CH4 to moles or moles CH4 to grams. Molecular weight calculation: 12.0107 + 1.00794*4.
How many atoms are in CH4?
When we talk about CH4 it is basically a combination of 1 carbon and 4 hydrogen atoms. However, to form this compound the central atom carbon which has 4 valence electrons obtain more electrons from 4 hydrogen atoms to complete its octet. Why is oxygen never the central atom? 1. With only two atoms in the molecule, there is no central atom.
Why is CH4 a molecule?
Methane is a tetrahedral molecule with four equivalent C–H bonds. Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H.
Is CH4 an example of a molecule?
H2O H 2 O (water) and CH4 C H 4 (methane) are both examples of a) molecules. Molecules are formed when atoms of elements bond together. Both water and methane are examples of molecules that contain covalent bonds. Covalent bonds are the type of bonds in which atoms share electron pairs.
Is a formula unit a molecule?
It is also helpful in balancing the equations, doing calculations and finding the molecular formula of the compound whereas the term formula unit is entirely different from the term molecule. A formula unit is a term used for defining the whole lesser number of ratios of ions in an ionic compound.
What is CH4 formula?
CH₄Methane / Formula
Is CO2 a molecule?
Carbon dioxide is a molecule with the molecular formula CO2. Carbon dioxide, CO2, is a colorless gas. It is made of two oxygen atoms covalently bonded to one carbon atom.
What type of molecular bond is CH4?
The methane, CH4, molecule composition shows single covalent bonds. Covalent bonding entails electrons being exchanged. The four hydrogen atoms share one electron each with the carbon atom in the methane molecule.
Is H2O a molecule or formula unit?
Each molecule of water contains one atom of oxygen and two atoms of hydrogen. So, H2O is its formula. This can be more specifically called a molecular formula. In ionic compounds such as table salt, NaCl, the atoms (as ions) do not bond to specific neighbors.
What is an example of a formula unit?
Table salt, for example, has a formula unit of NaCl because the charge of a sodium ion (Na) is +1 and the charge of a chloride ion (Cl) is -1.
What are formula units?
A formula unit in chemistry is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound.
Is methane a molecular compound?
MethaneMethane / IUPAC ID
Which statement is correct about CH4?
Option 2 is correct. CH4 is the chemical formula for methane. It is a symmetrical molecule which shows tetrahedral geometry. It is also an overall non polar molecule.
Is oxygen a molecule?
Oxygen is found naturally as a molecule. Two oxygen atoms strongly bind together with a covalent double bond to form dioxygen or O2. Oxygen is normally found as a molecule. It is called dioxygen.
Why is CH4 a nonpolar molecule?
CH4 is a nonpolar molecule as it has a symmetric tetrahedral geometrical shape with four identical C-H bonds. The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges to be almost zero.
Is H2 a molecule?
The hydrogen molecule, H2 is pulled apart to form two H+ (hydrogen cations) and two electrons.
What type of molecule is CH4 polar or nonpolar?
non polarCH4 is non polar.
Is methane gas harmful to humans?
Methane alone is non-toxic but can become deadly when mixed with other gases. Methane displaces oxygen to induce asphyxiation. It can cause dizzine...
What is the main cause of methane gas?
Methane is released during coal, natural gas, and oil production and transportation. Methane emissions are also caused by livestock and other farmi...
Why is methane gas bad for the environment?
For example, if methane spills into the air before being used from a leaky pipe – it absorbs the heat from the sun, warming up the atmosphere. It’s...
Is methane a fossil fuel?
Fossil fuels range from volatile materials with low carbon-to-hydrogen ratios (like methane), to liquids (like petroleum), to almost pure carbon-co...
Can Methane be extracted from the atmosphere?
The bacteria can then be used to extract methane from the air. Because of its intense effectiveness as a greenhouse gas, Boucher and Folberth claim...
What is CH4 in chemical terms?
CH4 is the chemical formula for Methane. Four Hydrogen atoms covalently bonded to one Carbon atom. Covalently bonded substances exist as individual particles called molecules.
How many hydrogen atoms are in CH4?
CH4 states that there are 4 hydrogen atoms (H4) covalently bonded (covalent bond meaning an atom shares valence electrons with another atom in order to become more stable) to a single carbon atom (C) and therefore acts as one entity until these bonds are broken. The definition of a molecule being a group of atoms bonded together, makes CH4 a molecule.
What is a group of atoms bonded together?
a molecule is, a group of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction.
What is CH4 gas?
It is a molecule called methane or natural gas.CH4 is methane, a colorless, odorless gas. It is the simplest of hydrocarbons.
What type of bond is found in methane?
The chemical bonds found within methane are classified as covalent bonds. A covalent bond is formed when two or more atoms share one or more electrons located in the outermost energy level, also known as the valenceshell. The valence electrons from each donor atom form pairs through overlap of their electron clouds to create a covalent bond.
How do covalent bonds form in methane?
For methane the covalent bonds form from the sharing of a single electron from each hydrogen with the four unpaired valence electrons of a single carbon atom. The hydrogen atoms are arranged around the central carbon atom in a geometry known as a tetrahedral geometry. Tetrahedral geometry means that, if you drew a line to connect three hydrogen atoms on the same side of the molecule, you would have a pyramid with four triangular faces.
Is CH4 a gas?
CH4 is methane, a colorless, odorless gas. It is the simplest of hydrocarbons, as it contain only 1 carbon atom. Furthermore, it’s electronic and geometric configurations are both tetrahedral. It’s very stable, containing 4 carbon-hydrogen polar covalent bonds. However, it is without a permanent dipole moment due to its geometry - the individual bond moments sum vectorially to zero.
What is the formula weight of a compound?
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction.
How to calculate molar mass?
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
How to find the formula weight?
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Is molar mass the same as molecular mass?
This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight ...
What is the rate constant for the vapor phase reaction of methane with photochemically produced hydroxyl radical?
The rate constant for the vapor-phase reaction of methane with photochemically-produced hydroxyl radicals is 6.86X10-15 cu cm/molecule-sec at 25 °C (1). This corresponds to an atmospheric half-life of about 4 years at an atmospheric concentration of 5X10+5 hydroxyl radicals per cu cm (2). Methane is not expected to undergo hydrolysis in the environment due to the lack of functional groups that hydrolyze under environmental conditions (3). Methane does not contain chromophores that absorb at wavelengths >290 nm (3) and, therefore, is not expected to be susceptible to direct photolysis by sunlight (SRC).
What is activated charcoal used for?
Activated charcoal is used as a gastric decontamination agent in emergency clinical setting s in case of poison or medication overdose. Studies show that early administration of one dose of activated charcoal can adsorb poison in the stomach and reduce absorption while it also works long after ingestion, by interruption of enterohepatic and enterovascular cycling of poison.
Is a mononuclear parent hydride a compound?
It is a mononuclear parent hydride, a one- carbon compound, a gas molecular entity and an alkane. It is a conjugate acid of a methanide. Natural gas, refrigerated liquid (cryogenic liquid) appears as a flammable liquefied gaseous mixture of straight chain hydrocarbons, predominately methane. CAMEO Chemicals.
Is methane a gas?
Methane is the principal constituent of natural gas (1,3); natural gas from America is approximately 85% methane (4). Emissions of geothermal steam may release methane to the environment (1,5). Methane is a constituent of petroleum gases (6).