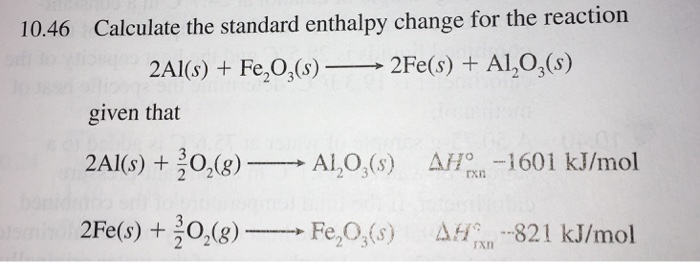
Why is Q equal to enthalpy?
This expression is consistent with our definition of enthalpy, where we stated that enthalpy is the heat absorbed or produced during any process that occurs at constant pressure. At constant pressure, the change in the enthalpy of a system is equal to the heat flow: ΔH=qp.
What is the value Q for enthalpy?
0:083:355.1 Calculating ΔH using q = mcΔT (SL) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo in this video we'll be using the equation Q equals MC delta T where Q is heat in joules M is massMoreSo in this video we'll be using the equation Q equals MC delta T where Q is heat in joules M is mass in grams and C is the specific heat capacity.
How are Q and H related?
2:413:57The difference between H and q - YouTubeYouTubeStart of suggested clipEnd of suggested clipIt's actually transitioning from one state or from one object to another but this is gonna be energyMoreIt's actually transitioning from one state or from one object to another but this is gonna be energy less as I they're gonna be gained. Or lost whereas the H is going to be a total amount of the heat.
Are Q and Delta u the same?
Q is the net heat transferred into the system—that is, Q is the sum of all heat transfer into and out of the system. W is the net work done on the system....Δ U \Delta U ΔU (change in internal energy)Q (heat)W (work done on gas)is + if temperature T increasesis + if heat enters gasis + if gas is compressed2 more rows
Is Q the same as energy?
q value is not the same as 'kinetic energy change'. q is energy exchanged in the form of heat. When system intakes heat, its internal energy can change or remain the same. Internal energy is actually Total Energy = Kinetic Energy + Potential Energy.
What does Q represent in entropy?
One useful way of measuring entropy is by the following equation: DS = q/T (1) where S represents entropy, DS represents the change in entropy, q represents heat transfer, and T is the temperature. Using this equation it is possible to measure entropy changes using a calorimeter. The units of entropy are J/K.
What is the difference between enthalpy and heat Q?
The key difference between enthalpy and heat is that enthalpy describes the amount of heat transferred during a chemical reaction at constant pressure whereas heat is a form of energy. Furthermore, enthalpy is a function of the state, whereas heat isn't since heat is not an intrinsic property of a system.
How is entropy related to Q?
The change in entropy (delta S) is equal to the heat transfer (delta Q) divided by the temperature (T). An example of a reversible process would be ideally forcing a flow through a constricted pipe.
What formula is Q MC ∆ T?
The amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcΔT, where m is the mass of the sample, c is the specific heat, and ΔT is the temperature change.
Is Q energy or heat?
Scientists define heat as thermal energy transferred between two systems at different temperatures that come in contact. Heat is written with the symbol q or Q, and it has units of Joules ( Jstart text, J, end text).
What is the relationship between Q and Delta T?
The amount of heat transferred delta Q is proportional to the temperature difference delta T between the objects and the heat capacity c of the object. The heat capacity is a constant that tells how much heat is added per unit temperature rise.
Is Q and K the same?
It is important to understand the distinction between Q and K. Q is a quantity that changes as a reaction system approaches equilibrium. K is the numerical value of Q at the "end" of the reaction, when equilibrium is reached.
What is Q enthalpy?
q is the amount of heat transferred to a system whereas is used to describe the change in enthalpy. Enthalpy is the total potential energy of a system, which is associated with the heat transferred to/from a system (q).
What is the Q value in Q MC ∆ T?
0:009:19Specific Heat Capacity (q=mC∆T) Examples, Practice Problems, Initial ...YouTubeStart of suggested clipEnd of suggested clipAnd that has to be in joules m is the mass and the units for that's grams c is the specific heatMoreAnd that has to be in joules m is the mass and the units for that's grams c is the specific heat capacity and the units for that's joules per gram times degrees kelvin or it can also be joules.
What is Q in Q MC ∆ T?
The amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcΔT, where m is the mass of the sample, c is the specific heat, and ΔT is the temperature change.
What is Q UA ∆ T?
From the overall equation, the total heat transferred per unit time is given by. q = UA∆Tm where ∆Tm is the mean temperature difference. But the total heat transferred per unit is also: q = cpG (T1 –T 2)
What is the law of thermodynamics for gas?
Let's consider the first law of thermodynamics for a gas. For a system with heat transfer Q and work W, the change in internal energy E from state 1 to state 2 is equal to the difference in the heat transfer into the system and the work done by the system: E2 - E1 = Q - W. The work and heat transfer depend on the process used to change the state.
What is thermodynamics in science?
Thermodynamics is a branch of physics which deals with the energy and work of a system. Thermodynamics deals only with the large scale response of a system which we can observe and measure in experiments.
What is the Cp of specific heat?
The specific heat capacity cp is called the specific heat at constant pressure and is related to the universal gas constant of the equation of state. This final equation is used to determine values of specific enthalpy for a given temperature. Enthalpy is used in the energy equation for a fluid. Across shock waves , the total enthalpy of the gas remains a constant.
What are the properties of a gas?
The state of a gas is defined by several properties including the temperature , pressure , and volume which the gas occupies. From a study of the first law of thermodynamics, we find that the internal energy of a gas is also a state variable, that is, a variable which depends only on the state of the gas and not on any process that produced that state. We are free to define additional state variables which are combinations of existing state variables. The new variables often make the analysis of a system much simpler. For a gas, a useful additional state variable is the enthalpy which is defined to be the sum of the internal energy E plus the product of the pressure p and volume V . Using the symbol H for the enthalpy:
How many moles of pure water are in the initial state of a system?
Strictly speaking, that Δ H assumes that the initial state consists of 2 moles of pure H X 2 and 1 mole of pure O X 2, each at 298 K and 1 bar, and the final state consists of 2 moles of pure liquid water at the same temperature and pressure. Since enthalpy is a state function independent of path, this enthalpy change is independent of any and all processes used for transitioning the system from state 1 to state 2.
Can H X 2 and O X 2 react?
Since, at this pressure, H X 2 and O X 2 behave essentially as ideal gases (and their mixing is ideal), the gases can be mixed first and then allowed to react at constant pressure. In this case, the heat added to the system with be equal to the stated Δ H (in this case negative).
Is a heat engine a cycle?
The process is a cycle, meaning the initial and final states are the same, so a cycle of a heat engine has zero enthalpy change. However, the entire point is that the heat engine converts heat into work, so the heat exchange during a cycle is not zero.
Is heat a function of enthalpy?
Enthalpy and heat are entirely different things. Enthalpy is a function of state. If you know the state of a system, you know its enthalpy. If you know the starting and ending states of a process, you can find the enthalpy change. Heat, on the other hand, is an inexact differential.
Is heat a differential?
Heat, on the other hand, is an inexact differential. Knowing the initial and final states of a process is not enough information to tell you the heat transfer. Instead, the heat transfer depends on the particular path taken between the states. For a simple example of why this is important, consider a heat engine.
Is heat exchanged under constant pressure?
That is why the heat exchanged is equal to the enthalpy change only under constant pressure. We need to give extra information (such as that the pressure is constant) to know the path taken during the process in order to say anything meaningful about heat exchange.
How to calculate changes in enthalpy?
You can calculate changes in enthalpy using the simple formula: ∆H = Hproducts− Hreactants
What is change in enthalpy?
Changes in enthalpy describe the energy input or output resulting from chemical reactions, and learning to calculate them is essential for any higher-level che mistry student.
What is the enthalpy of fusion?
When a substance changes from solid to liquid, liquid to gas or solid to gas, there are specific enthalpies involved in these changes. The enthalpy (or latent heat) of melting describes the transition from solid to liquid (the reverse is minus this value and called the enthalpy of fusion), the enthalpy of vaporization describes ...
What is the enthalpy of sodium chloride?
The addition of a sodium ion to a chloride ion to form sodium chloride is an example of a reaction you can calculate this way. Ionic sodium has an enthalpy of −239.7 kJ/mol, and chloride ion has enthalpy −167.4 kJ/mol. Sodium chloride (table salt) has an enthalpy of −411 kJ/mol. Inserting these values gives:
What is the end product of a reaction if you start with six moles of carbon combined with three of hydrogen?
One example is if you start with six moles of carbon combined with three of hydrogen, they combust to combine with oxygen as an intermediary step and then form benzene as an end-product. Hess’ law states that the change in enthalpy of the reaction is the sum of the changes in enthalpy of both parts.
What is the enthalpy of melting water?
For water, the enthalpy of melting is ∆Hmelting= 6.007 kJ/mol. Imagine that you heat ice from 250 Kelvin until it melts, and then heat the water to 300 K. The enthalpy change for the heating parts is just the heat required, so you can find it using:
What is the enthalpy change of a reaction?
The enthalpy change of a reaction is the amount of heat absorbed or released as the reaction takes place, if it happens at a constant pressure. You complete the calculation in different ways depending on the specific situation and what information you have available. For many calculations, Hess’s law is the key piece of information you need to use, ...
How does the internal energy of a system affect the heat gained or lost by the system?
The sign convention for the relationship between the internal energy of a system and the heat gained or lost by the system can be understood by thinking about a concrete example, such as a beaker of water on a hot plate. When the hot plate is turned on, the system gains heat from its surroundings. As a result, both the temperature and the internal energy of the system increase, and E is positive. When the hot plate is turned off, the water loses heat to its surroundings as it cools to room temperature, and E is negative.
Why is the difference between E and H small?
The difference between E and H for the system is small for reactions that involve only liquids and solids because there is little if any change in the volume of the system during the reaction. The difference can be relatively large, however, for reactions that involve gases, if there is a change in the number of moles of gas in the course of the reaction.
How does internal energy relate to work?
The relationship between internal energy and work can be understood by considering another concrete example: the tungsten filament inside a light bulb. When work is done on this system by driving an electric current through the tungsten wire, the system becomes hotter and E is therefore positive. (Eventually, the wire becomes hot enough to glow.) Conversely, E is negative when the system does work on its surroundings.
What is the branch of science that deals with the relationship between heat and other forms of energy?
Thermodynamics is defined as the branch of science that deals with the relationship between heat and other forms of energy, such as work. It is frequently summarized as three laws that describe restrictions on how different forms of energy can be interconverted.
What is the internal energy of a system?
The internal energy of a system can be understood by examining the simplest possible system: an ideal gas. Because the particles in an ideal gas do not interact, this system has no potential energy. The internal energy of an ideal gas is therefore the sum of the kinetic energies of the particles in the gas.
What is the first law of thermodynamics?
The first law of thermodynamics can be captured in the following equation, which states that the energy of the universe is constant. Energy can be transferred from the system to its surroundings, or vice versa, but it can't be created or destroyed. A more useful form of the first law describes how energy is conserved.
Why is the volume of a reaction not constant?
When this is done, the volume of the system is not constant because gas can either enter or leave the container during the reaction.
What is the enthalpy change?
An enthalpy change is approximately equal to the difference between the energy used to break bonds in a chemical reaction and the energy gained by the formation of new chemical bonds in the reaction. It describes the energy change of a system at constant pressure. Enthalpy change is denoted by ΔH. At constant pressure, ΔH equals ...
How is entropy related to heat?
In an exothermic reaction, the entropy of the surroundings increases. As heat is evolved, the energy imparted to the system increases disorder. In an endothermic reaction, the external entropy decreases.
What happens to the external entropy of an endothermic reaction?
In an endothermic reaction, the external entropy decreases. As heat is absorbed by a process or reaction, the kinetic energy of molecules in the surroundings decreases, which tends to reduce reduce disorder. Helmenstine, Anne Marie, Ph.D. "Enthalpy Change Definition in Science.".
What is Enthalpy?
When a process takes place at constant pressure, the heat absorbed or released is equal to the Enthalpy change. Enthalpy is sometimes known as “heat content”, but “enthalpy” is an interesting and unusual word, so most people like to use it. Etymologically, the word “entropy” is derived from the Greek, meaning “turning” and “enthalpy” is derived from the Greek meaning “warming”. As for pronunciation, Entropy is usually stressed on its first syllable, while enthalpy is usually stressed on the second.
Where does the word "entropy" come from?
Etymologically, the word “entropy” is derived from the Greek, meaning “turning” and “enthalpy” is derived from the Greek meaning “warming”. As for pronunciation, Entropy is usually stressed on its first syllable, while enthalpy is usually stressed on the second.
What is the sum of the internal energy and the product of pressure and volume?
Enthalpy ( H) is the sum of the internal energy (U) and the product of pressure (P) and volume (V).
What is the branch of physics that deals with the relationship between heat and other forms of energy?
Thermodynamics is the branch of physics that deals with the relationships between heat and other forms of energy. In particular, it describes how thermal energy is converted to and from other forms of energy and how it affects matter. First law of thermodynamics: one of the most fundamental laws of nature is the conservation of energy principle.
What is the second law of thermodynamics?
The second law of thermodynamics: energy has quality as well as quantity, and actual processes occur in the direction of decreasing quality of energy. Whenever there is an interaction between energy and matter, thermodynamics is involved. Some examples include heating and air-conditioning systems, refrigerators, water heaters, etc.
Is enthalpy measured directly?
Enthalpy is not measured directly, however, the change in enthalpy (ΔH) is measured, which is the heat added or lost by the system. It is entirely dependent on the state functions T, p and U.
