What are the two allotropes of carbon?
In the crystalline form, diamond and graphite are the two allotropes of carbon while the amorphous forms of carbon are coal, coke, petroleum coke, gas carbon, etc. In this article, we will learn more about the allotropes of carbon: diamond, graphite, and fullerenes. What are allotropes? Why is diamond hard? What are allotropes?
Which of the following is an amorphous carbon?
Amorphous carbon is an allotrope of carbon. It is free and reactive carbon that does not have any crystalline structure. It is a solid allotropic form of carbon. Coke, coal, charcoal, lamp black, gas carbon, carbon black, etc. are amorphous forms of carbon.
Is Coke an amorphous allotropic form of carbon?
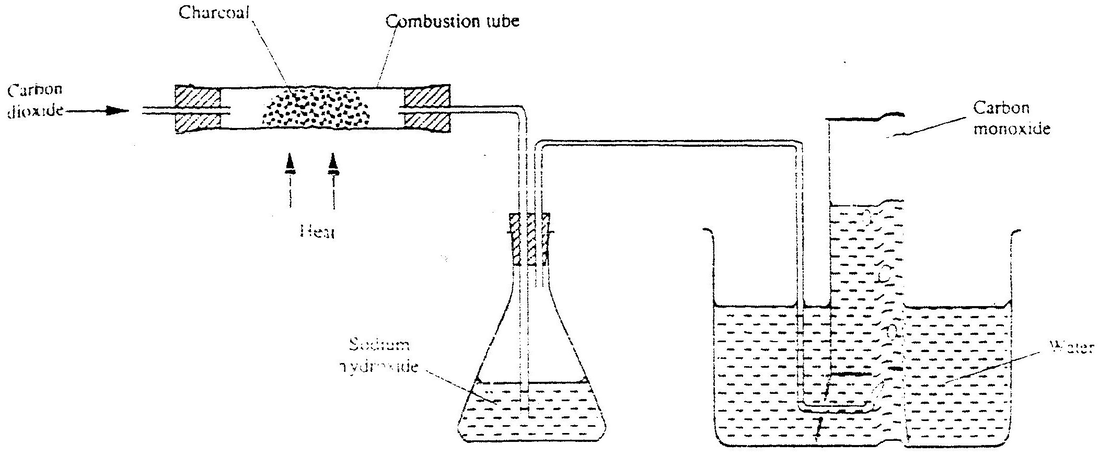
Ans: Yes, coke is an amorphous allotropic form of Carbon. Coke is obtained by destructive distillation of coal and contains about 80 to 95 Carbon. Q.2. What are Allotropes?
What are crystalline allotropes?
Crystallines are those allotropes that have a particular shape or geometry of a carbon atom. Crystalline allotropes have a particular shape so it has different physical property like refracting index, melting point, density, etc. But the chemical property remains the same. All the carbon in the diamond is sp3 hybridized.

Which are the allotropes of carbon?
Diamond, graphite and fullerenes (substances that include nanotubes and 'buckyballs' , such as buckminsterfullerene) are three allotropes of pure carbon.
Why coal is not an allotrope of carbon?
Carbon is a pure element. Coal is a mixture of many elements and compounds (including Carbon) but, mostly has not yet been formed into carbon by combustion. Was this answer helpful?
What are the 4 main allotropes of carbon?
Use the accompanying fact sheet and differentiated flash card activity to explore the different properties and uses of four allotropes of carbon – diamond, graphite, graphene and buckminsterfullerene.
Is charcoal a crystalline allotrope of carbon?
Hence, the crystalline allotrope of carbon is Graphite. Amorphous compounds are compounds that have no ordered manner of arrangement of atoms. Amorphous forms of carbon are coal, coke, charcoal, lamp black, and gas carbon.
Is charcoal a diamond?
Diamonds are indeed just crystalline carbon (charcoal is another form of carbon as is graphite (e.g. pencil lead)). Unfortunately, carbon only crystalizes at tremendous pressures and high temperatures.
Which is not allotropes of carbon?
Soot. The explanation for the correct option: Option (b): Soot is a black powder-like substance, which is not an allotrope of carbon.
What are the 5 allotropes of carbon?
Diamond, Graphite, Fullerene, and carbon nanotubes are prominent allotropes of carbon.
Which is not a form of carbon?
So, we have seen that microtubule is the only option which is not an allotrope of carbon. Hence, the answer to the given question is option (C).
How many allotropes of carbon are present in the world?
Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).
What are the 3 crystalline allotropes of carbon?
Summary. Different forms, or allotropes, of carbon are diamond, graphite, and fullerenes.
Is charcoal crystalline or amorphous?
amorphous carbon6.3. 4 Charcoal. Charcoal is normally obtained from the burning of wood, peat, bones, cellulose, or other carbonaceous substances with little or insufficient air. It is an amorphous carbon in the form of highly porous microcrystalline graphite.
Which are the two crystalline allotropes of carbon?
In addition to diamond, there are two other well-known crystalline allotropes of carbon, lonsdaleite and graphite. These two allotropes both have a hexagonal structure, although lonsdaleite is close packed, as described below, while graphite is not. However, their formation and properties are entirely different.
Is carbon a diamond or coal?
Coal and diamonds are both composed of carbon. The two materials differ in their microscopic arrangement of atoms, and that leads to quite a difference in appearance, conductivity, hardness and other properties.
Is coal a carbon?
Coal is a sedimentary deposit composed predominantly of carbon that is readily combustible. Coal is black or brownish-black, and has a composition that (including inherent moisture) consists of more than 50 percent by weight and more than 70 percent by volume of carbonaceous material.
What's the difference between diamond and coal?
The chemical difference is identical, but physically, diamond molecules are stacked neatly into uniform lattices, which is why they are so hard and crystal clear. Coal molecules, by comparison, are randomly stacked, which gives coal its colour and the fact that it burns and can easily be broken into smaller pieces.
What is the difference between coal and graphite?
Although both Graphite and Charcoal are have a carbon base, they do have quite distinct differences. One of their most noticeable differences is their finish. While charcoal gives a rich, matte black finish, graphite will always remain slightly reflective and metallic.
What are Allotropes?
The phenomenon of the existence of an element in two or more forms has different physical properties, but identical chemical properties are called...
Is coke an Allotrope of Carbon?
Yes, coke is an amorphous allotropic form of Carbon. Coke is obtained by destructive distillation of coal and contains about 80 to 95 Carbon.
Which is the hardest Allotrope of Carbon?
Diamond is the hardest Allotrope of Carbon.
What is the purest form of Carbon?
Fullerene is the purest form of Carbon because it does not have dazzling edges or surface bonds that attract other atoms as in the case of graphite...
How is fullerene obtained?
Fullerene is obtained by heating graphite in an electric arc in an inert gas such as helium or argon when a sooty material is formed by condensatio...
Crystalline
Crystallines are those allotropes that have a particular shape or geometry of a carbon atom. Crystalline allotropes have a particular shape so it has different physical property like refracting index, melting point, density, etc. But the chemical property remains the same.
Diamond
All the carbon in the diamond is sp3 hybridized. It consists of an innumerable number of tetrahedral units and the distance between lattices is 1.54Å. It Has thermal conductivity but not electricity. Dimond burns by oxygen to produce carbon dioxide at 800-9000C as it is another form of carbon
Graphite
Only graphite is a non-metal which can conduct electricity. Graphite consists of two adjacent parallel layers of C-C hexagon unit having a distance of 3.35 Å. The layers are weakly held by the van der Waal’s force so one layer can slide over another, for this reason, graphite is soft and lubricating.
Fullerene
Fullerene can behave as a superconductor even at very low temperatures like 10-40K. Different types of Fullerenes are listed below.
Amorphous
Amorphous is that allotrope of carbon that does not have a particular shape or lattice structure. Amorphous has also lattice structures but they are disorganized so they do not adopt a particular shape or structure. Their physical property is different but has the same chemical property.
Charcoal
Charcoal is used as gun powder and black paint. It is also used as fuel. Now various type of charcoal are discussed below,
Lamp black or Carbon black
The nature of lamp black allotrope is black sooty smoke, which usually comes out after the condensation. Lamp black is the purest form of all. It is a very poor conductor of heat as well as electricity. When methane is burned then lamp black is also produced. It is is used for shoe polish and black paints.
What are Allotropes?
Allotropy is the occurrence of an element existing in two or more forms with distinct physical qualities but similar chemical properties, and the different forms are known as allotropes.
What is the bond between carbon and graphite?
Each Carbon atom in graphite is in a state of sp2 hybridization, which means it is covalently linked to three other carbon atoms in the same plane. Planar hexagonal rings are produced as a result. The length of the Carbon-Carbon bond in this ring is 142pm.
How many electrons does carbon have?
Carbon has an atomic number of 6, which indicates that the electronic configuration of the carbon atom is 2,4. Because a carbon atom’s outermost shell has four electrons, it shares those electrons and reaches the inert gas state. As a result, the carbon atom creates covalent connections with other atoms.
What is the main unadulterated type of carbon?
Fullerenes are the main unadulterated type of Carbon since they don’t have astonishing edges or surface securities that draw in different iotas, as on account of graphite or jewel.
How many electrons are needed to make hexagonal rings in graphite?
The fourth valence electron of each Carbon is free to travel since only three electrons of each Carbon are required to make hexagonal rings in graphite. As a result, graphite is a strong heat and electrical conductor.
What is the organization of jewel?
Jewel has a three-dimensional organization of carbon molecules combined through solid covalent bonds. Every Carbon iota is in the condition of sp 3 hybridisation and connected tetrahedrally to four adjoining Carbon molecules. This organization stretches out into three measurements. All Carbon-Carbon (C–C) bonds are equivalent and equivalent to 154 pm, and every C–C–C bond point is 109 ∘ 28′.
What is the bonding number of carbon?
Bonding in Carbon: Carbon has an atomic number of 6, which indicates that the electronic configuration of the carbon atom is 2,4. Because a carbon atom’s outermost shell has four electrons, it shares those electrons and reaches the inert gas state. As a result, the carbon atom creates covalent connections with other atoms.
What are Allotropes of Carbon?
Carbon is one of the elements which shows allotropy. The allotropes of carbon can be either amorphous or crystalline (Diamond, Graphite).
How are carbon allotropes prepared?
They are spheroidal molecules having the composition, C2n, where n ≥ 30. These carbon allotropes can be prepared by evaporating graphite with a laser.
What is graphite used for?
One of the most important properties of graphite is that it is used as a dry lubricant for machines at high temperature where we cannot use oil. Graphite is used to make crucibles which have the property that they are inert to dilute acids as well as to alkalis.
What are the C atoms?
The C atoms are bonded in flat hexagonal lattices (graphene), which are then layered in sheets. Linear acetylenic carbon (Carbyne) Amorphous carbon. Fullerenes, including Buckminsterfullerene, also known as “buckyballs”, such as C60. Carbon nanotubes: Allotropes of carbon with a cylindrical nanostructure.
Why is carbon an allotrope?
Carbon due to its capability of having variable oxidation states or coordination number makes carbon one of the few elements to have multiple numbers of allotropic forms. Carbon’s ability to catenate is another contributing factor. Thus, it leads to the formation of various allotropes of carbon.
What is the phenomenon by which an element can exist in more than one physical state?
The phenomenon by which an element can exist in more than one physical state is called allotropy. The allotropes of carbon can be categorized into two: Amorphous Carbon Allotropes. Crystalline Carbon Allotropes.
How many carbon atoms are in a sigma bond?
Out of four carbon atoms three forms sigma bonds whereas the fourth carbon forms pi-bond. The layers in graphite are held together by Vander Waal forces.
What are allotropes?
Allotropes or allotropy may be defined as the existence of a chemical element in one or more physical forms occurring in the same physical state. Allotropes may show different physical or chemical states depending upon the atom arrangement or the number of existent atoms.
Diamond
Diamond is the crystalline form of carbon. It is one of the purest forms of crystalline carbon present on earth.
Graphite
Graphite is one of the most commonly found naturally occurring allotropes of carbon.
Fullerenes
Unlike graphite and diamond, fullerene doesn’t have any dazzling edges or surface bonds to attract atoms hence it is considered the only purest form of carbon.
Why is diamond hard?
Each carbon has four electrons on its outermost shell. In the case of diamonds, these electrons are bonded with other carbon atoms resulting in a strong chemical bond. The presence of this bond creates an extremely rigid tetrahedral crystal. In simple words, the tightly arranged structure makes diamond the hardest substance on the planet.
Uses of allotropes of carbon
Each allotrope of carbon has its unique physical and chemical properties. Based on their properties each allotrope of carbon is used commercially for different purposes.
Things to remember
Allotrope refers to one or more forms of an element occurring in the same state of matter. Each allotrope of an element has distinct physical and chemical properties.
What is an allotrope of carbon?
Allotropy is a property for which a basic element may exist in two or more forms leaving its original chemical properties unchanged. Some elements may have two or more different forms without changing their basic chemical properties. Those different forms of the element are called allotropes. Carbon is found in abundance in nature as an element in the free state and as a compound when combined with other elements. This element has the ability to form many different compounds because each carbon element can have four chemical bonds with other elements. That is, carbon is an element that has two or more different forms or shows allotropy (1).
What is Buckminsterfullerene made of?
Buckminsterfullerene is a crystalline allotrope of carbon. Its molecule is made up of 60 carbon atoms therefore has a formula is C₆₀. The structure of the C₆₀ molecule is like a football. Its molecular structure consists of a ring made up of five and six carbon atoms, forming a multi-layered molecule.
How is lonsdaleite formed?
Lonsdaleite is transparent. It is formed due to asteroidal impacts when meteorites with graphite hit the earth. This allotrope of carbon played a central role during the transformation of diamonds from graphite. Lonsdaleite is 58% stronger than diamond.
What is a single-walled carbon nanotube?
A single-walled carbon nanotube is hollow and cylindrical in shape. These are one of the allotropes of carbon that intersect between fullerene and graphene. The single-walled carbon nanotube is called Bucky tube. They have very high electrical conductivity. The single-walled carbon nanotubes have a low density.
What is the element carbon?
That is, carbon is an element that has two or more different forms or shows allotropy (1). 1. Diamond. Diamonds are a special form of an allotrope of carbon. It is a gemstone that is widely used in jewelry making. This gem is made from a single pure material carbon. The diamond is transparent.
What is the carbon in the air?
Carbon dioxide, a component of air, contains carbon. Petroleum and natural gas contain carbon in the form of hydrocarbons combined with hydrogen. Carbon as a mineral is in the form of carbonate in various compounds. Various plant and animal substances also contain carbon. Carbon resides in nature in various forms.
Why is carbon found in abundance?
This element has the ability to form many different compounds because each carbon element can have four chemical bonds with other elements .
What are the structures of carbon?
Larger scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).
What is the difference between graphite and diamond?
Between diamond and graphite: 1 Diamond crystallizes in the cubic system but graphite crystallizes in the hexagonal system. 2 Diamond is clear and transparent, but graphite is black and opaque. 3 Diamond is the hardest mineral known (10 on the Mohs scale ), but graphite is one of the softest (1–2 on Mohs scale ). 4 Diamond is the ultimate abrasive, but graphite is soft and is a very good lubricant. 5 Diamond is an excellent electrical insulator, but graphite is an excellent conductor. 6 Diamond is an excellent thermal conductor, but some forms of graphite are used for thermal insulation (for example heat shields and firebreaks). 7 At standard temperature and pressure, graphite is the thermodynamically stable form. Thus diamonds do not exist forever. The conversion from diamond to graphite, however, has a very high activation energy and is therefore extremely slow.
What are the different forms of carbon?
Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger scale structures of carbon include nanotubes, nanobuds and nanoribbons.
What happens when graphite loses its lubrication properties?
When a large number of crystallographic defects (physical) bind these planes together, graphite loses its lubrication properties and becomes pyrolytic carbon, a useful material in blood-contacting implants such as prosthetic heart valves .
How does graphite conduct electricity?
Graphite conducts electricity, due to delocalization of the pi bond electrons above and below the planes of the carbon atoms. These electrons are free to move, so are able to conduct electricity. However, the electricity is only conducted along the plane of the layers. In diamond, all four outer electrons of each carbon atom are 'localized' between the atoms in covalent bonding. The movement of electrons is restricted and diamond does not conduct an electric current. In graphite, each carbon atom uses only 3 of its 4 outer energy level electrons in covalently bonding to three other carbon atoms in a plane. Each carbon atom contributes one electron to a delocalized system of electrons that is also a part of the chemical bonding. The delocalized electrons are free to move throughout the plane. For this reason, graphite conducts electricity along the planes of carbon atoms, but does not conduct electricity in a direction at right angles to the plane.
Which is the most stable allotrope?
Although graphite is the most stable allotrope of carbon under standard laboratory conditions (273 or 298 K, at 1 atm), a recent computational study indicated that under idealized conditions ( T = 0, p = 0), diamond is the most stable allotrope by 1.1 kJ/mol compared to graphite.
How is glassy carbon prepared?
The preparation of glassy carbon involves subjecting the organic precursors to a series of heat treatments at temperatures up to 3000 °C. Unlike many non-graphitizing carbons, they are impermeable to gases and are chemically extremely inert, especially those prepared at very high temperatures. It has been demonstrated that the rates of oxidation of certain glassy carbons in oxygen, carbon dioxide or water vapor are lower than those of any other carbon. They are also highly resistant to attack by acids. Thus, while normal graphite is reduced to a powder by a mixture of concentrated sulfuric and nitric acids at room temperature, glassy carbon is unaffected by such treatment, even after several months.

What Are Allotropes of Carbon?
Graphite
- It is also a pure form of carbon. This allotrope of carbon is composed of flat two-dimensional layers of carbon atoms which are arranged hexagonally. It is a soft, black and slippery solid. This property of graphite persists because it cleaves easily between the layers. In each layer, each C atom is linked to three C atoms via a C-C covalent bond. ...
Diamond
- It is the purest crystalline allotrope of carbon. It has a number of carbons, linked together tetrahedrally. Each tetrahedral unit consists of carbon bonded to four carbon atoms which are in turn bonded to other carbons. This gives rise to an allotrope of carbon having a three-dimensional arrangement of C-atoms. ⇒ Also Read: Chemical Bonding Each carbon is sp3 hybridized and fo…
Other Carbon Allotropes
- Buckminsterfullerene
Buckminsterfullerene (C60) is also one of the allotropes of carbon. The structure of fullerene is like in a cage shape due to which it looks like a football. - Fullerenes
They are spheroidal molecules having the composition, C2n, where n ≥ 30. These carbon allotropes can be prepared by evaporating graphite with a laser. Unlike diamond, fullerenes dissolve in organic solvents. The fullerene C60 is called ‘Buckminster Fullerene’. The carbon ato…