
The color, shape, or other traits may be different, but the active ingredient for brands and generics are the same. You can also tell the difference by the name. The generic name is also the active ingredient, like the sedative diazepam for the brand Valium.
What is the difference between brand and generic name?
- The active ingredient is the same as in the name-brand drug.
- It has the same strength and directions for usage (tablet or injectable/oral or topical).
- The drug is manufactured under the same strict standards as the name-brand medicine.
What is an example of a generic name?
- Definition of generic vs brand name drugs
- Patents and Exclusivities
- Trade Name
- Manufactured by
- Animal & Clinical (Human) study for generic vs brand name drugs
- Price of generic vs brand name drugs Why generic drugs are cheaper?
- Appearance (Color, Shape, Size) of Generic vs brand-name drugs
- Name variation
- Excipients in generic vs brand name drugs
- Availability
Are generic drugs same as name brand?
Wednesday’s move by the Food and Drug Administration will allow pharmacists to ... compared to $340 to $520 for the same supply of brand-name Lantus.
What is brand name and generic name?
While A brand name drug is patent protected that disallow other company for selling generic versions of the same drug. Trade Name. A generic drug is marketed under the generic name of the drug. On the other hand, a brand name drug is marketed under a unique, proprietary name given by a pharmaceutical company. Application for USFDA approval
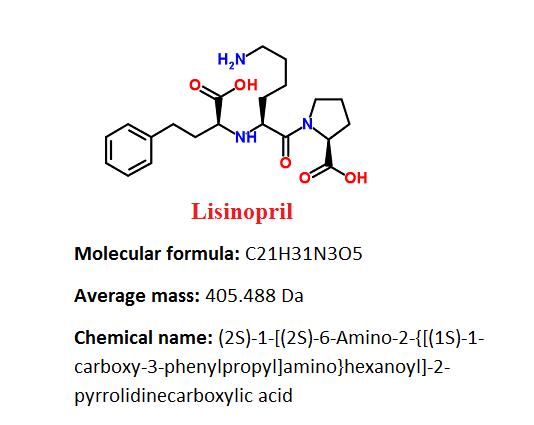
Is generic name chemical name?
Generic names are usually more complicated and harder to remember than brand names. Many generic names are a shorthand version of the drug's chemical name, structure, or formula.
Is generic name and scientific name the same?
Such a name is called a binomial name or a scientific name. The generic name or the initial part of the name highlights the genus to which an organism belongs to. The second part, or the specific name, identifies the exact species to which the organism falls under, within the genus.
What is called as generic names?
generic nameThe official nonproprietary name of a drug, under which it is licensed and identified by the manufacturer.The first name of the two-part Latin taxonomic name of an organism, which specifies the biological genus.A designation formerly used to indicate the class or type of a compound.
Is the generic name the official name?
The generic name is assigned, in the United States, by an official body—the United States Adopted Names (USAN) Council. The brand name is developed by the company requesting approval for the drug and identifies it as the exclusive property of that company.
Is paracetamol a generic name?
Thus, paracetamol/acetaminophen is the non-proprietary name (generic name) while Crocin/Metacin/Meftal/Tylenol etc. are brand names.
What is generic name and brand name?
Every medicine has two names: a brand name, which is given by the pharmaceutical company that markets the drug. a generic name, which is the drug's 'active ingredient' that makes it work.
Is Ibuprofen a generic name?
Ibuprofen is the generic name for Motrin and Advil, used for pain, fever and inflammation.
Is Tylenol a generic name?
Generic Name: acetaminophen This drug is used to treat mild to moderate pain (from headaches, menstrual periods, toothaches, backaches, osteoarthritis, or cold/flu aches and pains) and to reduce fever.
How are generic names written?
Accordingly, the generic name is the genus of the species and is written by starting with a capitalized letter. Both the generic name and the specific epithet are in italicized form.
Why do medicines have generic and brand names?
Companies take out exclusive rights called patents on each new drug they discover. If a company has a patent on a drug, only that company can market it under their brand name once it's been granted a licence. Once the patent expires, other manufacturers can market generic versions.
Is there a big difference between generic and brand name drugs?
There are only two main differences between generic and brand-name drugs: The inactive ingredients, such as flavoring or preservatives, may change. Generics generally cost less than brand-name versions.
What is meant by generic name and brand name explain with one example?
An example of a generic drug, one used for diabetes, is metformin. A brand name for metformin is Glucophage. (Brand names are usually capitalized while generic names are not.) A generic drug, one used for hypertension, is metoprolol, whereas a brand name for the same drug is Lopressor.
What is generic medicine?
Generic medications are a chemical copy of the original brand, with the same active ingredients. Generics are also available at a lower cost than brand-name medications. In fact, generic drugs cost 85 percent. Trusted Source.
Where are generic medications made?
Most active pharmaceutical ingredients (API) and generic medications are made outside the U.S. in countries like China, India, and various other countries.
What is a brand medication?
A brand medication is the “innovator” or pioneer, and gets patent and exclusivity protection so generics can’t compete right away. Generic medications must meet the same quality, strength, and purity standards as brands, so they have the same benefits and effects.
How much money do generics save?
are for generic medications, brand-name medications account for 74 percent. Trusted Source. of spending on medications in the U.S. Generics save Americans billions every year. In fact, generics saved U.S. consumers $253 billion. Trusted Source.
What are some examples of unsafe drugs?
Some examples of medications sold by potentially unsafe online pharmacies include: 1 birth control pills 2 antidepressants 3 finasteride (Proscar), for an enlarged prostate 4 bupropion (Wellbutrin), to treat symptoms of depression or seasonal affective disorder
What are inactive ingredients in generics?
The inactive ingredients are fillers, binders, colors, flavors, and preservatives. These ingredients don’t affect how the medication works, but sometimes you might have a reaction to one of these ingredients.
Why should I stay with a brand name?
You may also need to stay with a brand-name if you didn’t respond to the generic, your symptoms got worse, or you had an allergic reaction or side effect from an inactive ingredient. Doctors sometimes prefer not to switch medications that have a narrow therapeutic index (NTI), or safe range.
What does FDA mean when it says generic drugs are the same?
The FDA stipulates that the generic drug must be “pharmaceutically equivalent” to its brand-name version. This ensures that generic drugs have the same. Trusted Source. purity, strength, stability, ...
What is generic drug label?
The generic drug’s label is the same as the brand drug’s label. The generic drug does not deteriorate over time. The legal exclusivities or patents have expired. The drug company must submit an ANDA, which is an abbreviated new drug application. It states that the generic drug meets each standard.
Why are generic drugs less expensive?
Because they do not have to undergo the same human and animal studies as brand-name drugs, FDA-approved generic drugs are significantly less expensive. This price difference influences some people’s decision to choose generic drugs over brand-name drugs.
Can generic drugs be the same as brand names?
This includes their appearance. United States trademark laws do not allow generic drugs to look exactly the same as the equivalent brand- name drugs. However, the extent of these differences is regulated.
Can a generic drug be produced correctly?
The manufacturer can produce the generic drug both correctly and consistently. The generic drug has the same “active ingredient” as the brand drug. The correct amount of the active ingredient gets to the target area in the body. The ”inactive ingredients” in the generic drug are safe.
Do generic drugs affect cardiovascular disease?
However, another analysis found that generic drugs may not have the same clinical impact for cardiovascular conditions.
Do generic drugs have to go through the same testing?
Generic drugs use the same active ingredients that the branded drugs carried out testing for, so they do not have to conduct the same testing.
Do generic name drugs work the same way as brand-name drugs?
Some people hear “generic” and think of generic cereals that taste funny or get soggy too soon. They often don’t stack up against the original and are usually considered “knock-offs.” Generic drugs are different.
Are generic name drugs less effective?
No. Generic medications are just as effective as brand-name drugs. According to the FDA, drug makers must prove that generic medications can be substituted for brand-name drugs and offer the same benefits as their brand-name counterparts.
How much cheaper are generic name drugs?
Generic medications typically cost about 80% to 85% less than the same brand-name drug. According to 1 study, generic drugs saved the U.S. healthcare system $1.67 trillion from 2007 to 2016.
How do I tell them apart?
If you’re worried about picking up the wrong prescription, you can relax. Telling the difference between a generic and a brand-name drug isn’t too hard.
Are generic name drugs safe?
Yes. Generic drugs are heavily regulated and go through a rigorous review process before they’re approved.
Are there times when I should choose a brand-name drug over a generic name?
A generic drug may sound like an obvious choice when it comes to deciding which version of a medication to take—after all, it’s cheaper and does the exact same thing.
How do I get my insurance to pay for a brand-name drug over a generic name?
Your insurance company will often prefer that you use a generic over a brand-name medication. But what should you do if your doctor recommends that you take a brand-name?
Why do generic drugs cost less than brand names?
Generic drugs tend to cost less than their brand-name counterparts because generic drug applicants do not have to repeat animal and clinical (human) studies that were required of the brand-name medicines to demonstrate safety and effectiveness.
What is generic drug?
What are generic drugs? A generic drug is a medication created to be the same as an already marketed brand-name drug in dosage form, safety, strength, route of administration, quality, performance characteristics, and intended use.
Why do FDA inspectors go to the generic drug manufacturer's facility?
FDA scientists review those procedures, and FDA inspectors go to the generic drug manufacturer's facility to verify that the manufacturer is capable of making the medicine consistently and to check that the information the manufacturer has submitted to FDA is accurate.
How much less is generic medicine?
The reduction in upfront research costs means that, although generic medicines have the same therapeutic effect as their branded counterparts, they are typically sold at substantial discounts, an estimated 80 to 85% less, compared with the price of the brand-name medicine.
What is the label for generic medicine?
The container in which the medicine will be shipped and sold is appropriate. The label is the same as the brand-name medicine's label. The drug information label for the generic medicine should be the same as the brand-name label.
How much money did generic drugs save?
According to the IMS Health Institute, generic drugs saved the U.S. healthcare system nearly $2.2 trillion from 2009 to 2019 2. When multiple generic companies are approved to market a single product, more competition exists in the marketplace, which typically results in lower prices for patients.
When a medicine, generic or brand-name, is mass produced, is it mass produced?
When a medicine, generic or brand-name, is mass produced, very small variations in purity, size, strength, and other parameters are permitted. FDA limits how much variability is acceptable.
Why are generic medicines cheaper than brand names?
Generic medicines cost less than brand-name medicines because the manufacturers have not spent money on research and development of the medicine, or buying the rights to sell it.
Why do we need patents for medicine?
The patent is designed to allow the company to make enough profits to recover the money it spent developing the medicine, or on buying the rights to market it.
Is generic medicine safe?
Are generic medicines as effective and safe as brand-name medicines? Yes. Because they contain the same active ingredient and dose, they will work in the same way. Generic medicines can only be sold in Australia if they meet the same strict standards of quality, safety and effectiveness as the original.
What is the difference between generic and brand name?
Appearance (Color, Shape, Size) of Generic vs brand-name drugs. A generic drug may look different in shape, size, and color from relevant brand name drug. While a brand name drug has a unique look as design during product development. In the United States, trademark laws do not allow a generic drug to look exactly identical to other existing drugs ...
What is generic drug?
A generic drug is an off-patent pharmaceutical product that is manufactured by a pharmaceutical company in the same strength, dosage form, route of administration, safety, quality, performance characteristics, and intended use after expiring the patent of the relevant brand name drug (Innovator drug).
Why are animal studies not essential for generic drug applicants?
Animal and clinical (human) studies not essential for generic drug applicants to establish the safety and effectiveness of that generic drug. Lessen time and money required in drug product research and development because Innovator Company already discloses the excipients (Inactive ingredients) of that brand name drug.
Why are generic drugs cheaper?
Why generic drugs are cheaper? 1 Animal and clinical (human) studies not essential for generic drug applicants to establish the safety and effectiveness of that generic drug. 2 Lessen time and money required in drug product research and development because Innovator Company already discloses the excipients (Inactive ingredients) of that brand name drug. 3 No promotional budget required. 4 Does not required to buy the right to sell the generic version of the brand name drug. 5 After the expiration of a brand name drug, market competition between multiple generic companies typically results in a low price.
What is a brand name drug?
A Brand name drug is a pharmaceutical product that is developed and marketed under a patent or registered trademark by a pharmaceutical company. But it is approved after establishing the drug’s safety and effectiveness through animal and clinical (human) studies. Also, brand name drugs known as innovator drugs.
What are the similarities between generic and brand drugs?
A generic drugs/ medicine of a brand name drug must have the same: Active ingredient (s), Strength, Indications, Dosage form such as tablet or syrup or suspension or emulsion, Route of administration such as oral or IV,
How much money did generic drugs save?
According to the IMS Health Institute, in the United States healthcare system, generic drugs saved 1.67 trillion dollars from 2007 to 2016 [7]. Generic medicines are typically 20 to 90% cheaper than brand name drugs [8].
