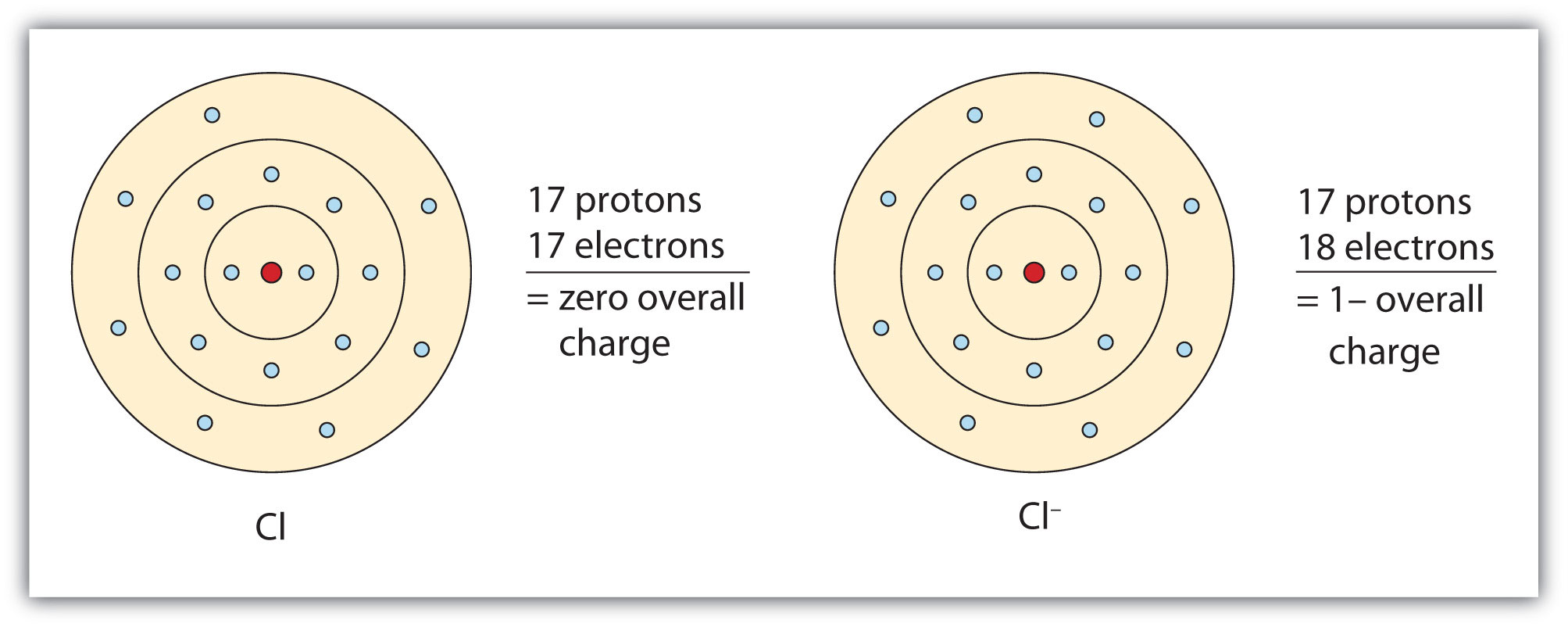
How many electrons does a neutral chlorine (Cl) atom contain?
Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and neutrons in the nucleus. If we subtract 17 (number of protons) from 35, we find there are 18 neutrons in a neutral chlorine atom's nucleus.
Is chlorine an acid or base or neutral?
Is chlorine a strong base? A strong acid like HCl donates its proton so readily that there is essentially no tendency for the conjugate base Cl – to reaccept a proton. Consequently, Cl – is a very weak base. Is bleach an acid or a base? Chlorine bleach is a base and is especially good at removing stains and dyes from clothes as well as ...
What is chlorine atom has 17 protons and 18 neutrons?
Chlorine has an atomic number of 17 and an atomic mass of 35.45, meaning that an atom of chlorine consists of 17 protons, 17 electrons, and 18 neutrons. As a member of the halogen family on the Periodic Table, chlorine is very reactive with metals and forms salts.
What does a neutral atom contain?
A neutral atom contains equal numbers of protons and electrons. But the number of neutrons within an atom of a particular element can vary. Atoms of the same element that have differing numbers of neutrons are called isotopes.
See more
What elements are neutral atoms?
When an atom has an equal number of electrons and protons, it has an equal number of negative (the electrons) and positive electric charges (the protons). As a result, the atom's total electric charge is zero, and it is said to be neutral. Therefore, all the elements in the periodic table are neutral atoms.
Which atom is not neutral?
Atoms which are not electrically neutral are called ions. One can collect electric charge by transferring electrons.
How many neutral atoms are in chlorine?
Chlorine has an atomic number of 17 and an atomic mass of 35.45, meaning that an atom of chlorine consists of 17 protons, 17 electrons, and 18 neutrons.
Are Cl ions neutral?
Since it has 1 more electron than protons, chlorine has a charge of −1, making it a negative ion.
What are the 3 neutral atoms?
A neutral, or stable, atom is composed of an equal amount of three components: protons, neutrons, and electrons.
What is an example of a neutral atom?
A neutral sodium atom, for example, contains 11 protons and 11 electrons. By removing an electron from this atom we get a positively charged Na+ ion that has a net charge of +1. Atoms that gain extra electrons become negatively charged. A neutral chlorine atom, for example, contains 17 protons and 17 electrons.
Why is cl2 neutral?
Cl2 consists of discrete molecules containing two chlorine atoms covalently bonded. The molecule has no net charge and is therefore neutral.
Why all atoms are neutral?
Every atom has no overall charge (neutral). This is because they contain equal numbers of positive protons and negative electrons. These opposite charges cancel each other out making the atom neutral.
What type of element is chlorine?
halogensChlorine is in group 17 of periodic table, also called the halogens, and is not found as the element in nature - only as a compound. The most common of these being salt, or sodium chloride, and the potassium compounds sylvite (or potassium chloride) and carnallite (potassium magnesium chloride hexahydrate).
Is Cl a neutral atom cation or anion?
These charged species are called ions....Cation vs anion chart.CationAnionChargePositiveNegativeElectrode attracted toCathode (negative)Anode (positive)Formed byMetal atomsNon-metal atomsExamplesSodium (Na+), Iron (Fe2+), Ammonium (NH4+)Chloride (Cl-), Bromide (Br-), Sulfate (SO42-)Aug 15, 2019
What type of ion is chlorine?
chlorine anionChloride Ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating fluid in and out of cells.
Which chloride is neutral?
Sodium chlorideSodium chloride, which is obtained by neutralization of hydrochloric acid and sodium hydroxide, is a neutral salt.
How do you know if an atom is not neutral?
Thus, if an atom contains equal numbers of protons and electrons, the atom is described as being electrically neutral. On the other hand, if an atom has an unequal number of protons and electrons, then the atom is electrically charged (and in fact, is then referred to as an ion rather than an atom).
Is electron a neutral atom?
Electrons are a type of subatomic particle with a negative charge. Protons are a type of subatomic particle with a positive charge. Protons are bound together in an atom's nucleus as a result of the strong nuclear force. Neutrons are a type of subatomic particle with no charge (they are neutral).
Why ions are not neutral?
The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons.
Is helium atom neutral?
There are two positive charges for the protons and two negative charges for the electrons. Therefore, helium doesn't have any net charge- it is a neutral atom.
How many electrons are in a neutral atom of chlorine?
Therefore, the number of electrons in neutral atom of Chlorine is 17. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
What is the electron configuration of chlorine?
Electron configuration of Chlorine is [Ne] 3s2 3p5. Possible oxidation states are +1,5,7/-1. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.
How many isotopes does chlorine have?
Chlorine has two stable isotopes, 35Cl and 37Cl. These are its only two natural isotopes occurring in quantity, with 35Cl making up 76% of natural chlorine and 37Cl making up the remaining 24%.The longest-lived radioactive isotope is 36Cl, which has a half-life of 301,000 years.
Why do neutrons stabilize the nucleus?
Neutrons stabilize the nucleus, because they attract each other and protons , which helps offset the electrical repulsion between protons. As a result, as the number of protons increases, an increasing ratio of neutrons to protons is needed to form a stable nucleus.
What is the mass number of isotopes of chlorine?
Mass numbers of typical isotopes of Chlorine are 35; 37.
What is the total electrical charge of the nucleus?
The total electrical charge of the nucleus is therefore +Ze , where e (elementary charge) equals to 1,602 x 10-19 coulombs. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A.
How are the chemical properties of a solid, liquid, gas, and plasma determined?
The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.