What is the difference between dehydration and dehydrogenation?
Dehydration is a reaction involving the removal or release of one or more molecules of water while dehydrogenation is a reaction involving the removal of one or more molecules of hydrogen. How do you compare the rate of dehydration?
What is oxidative dehydrogenation (ODH)?
The oxidative dehydrogenation (ODH) reaction of ethylbenzene to styrene in the presence of oxygen is one of the top ten industrial processes. From:Ordered Porous Solids, 2009
What is dehydrogenation of alcohols?
What is Dehydration of Alcohols? Alcohol upon reaction with protic acids tends to lose a molecule of water to form alkenes. These reactions are known as dehydrogenation or dehydration of alcohols. It is an example of an elimination reaction.
Is oxidative dehydrogenation of ethane an alternative to Endothermic dehydration?
The oxidative dehydrogenation (ODH) of ethane, which couples the endothermic dehydration of ethane with the strongly exothermic oxidation of hydrogen, would potentially be the most attractive alternative route because it avoids the need for excessive internal heat input, but also consumes valuable hydrogen.

What type of reaction is dehydrogenation?
Dehydrogenation is the chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem.
Is dehydration oxidation or reduction?
When an alcohol is dehydrated to form an alkene, one of the two carbons loses a C-H bond and gains a C-C bond, and thus is oxidized. However, the other carbon loses a C-O bond and gains a C-C bond, and thus is considered to be reduced. Overall, therefore, there is no change to the oxidation state of the molecule.
What is oxidative dehydrogenation?
Oxidative dehydrogenation (ODH) of ethane is an attractive, low energy, alternative route to ethene which could reduce the carbon footprint for its production, however, the commercial implementation of ODH requires catalysts with improved selectivity.
Is dehydrogenation an elimination reaction?
Alcohol upon reaction with protic acids tends to lose a molecule of water to form alkenes. These reactions are known as dehydrogenation or dehydration of alcohols. It is an example of an elimination reaction. Its rate varies for primary, secondary and tertiary alcohols.
What's the difference between dehydrogenation and oxidation?
Oxidation can be defined as the addition of oxygen to a molecule or the removal of hydrogen from a molecule. When an alkane is heated in the presence of an appropriate catalyst, it can be oxidized to the corresponding alkene in a reaction called a dehydrogenation reaction. Two hydrogen atoms are removed in the process.
Why is hydration not oxidation?
Addition or removal of water does not involve, by itself, an oxidation or a reduction reaction. The addition of water to an aldehyde to form a hydrate does not involve oxidation or reduction.
What is the other name of dehydrogenation?
It is the reverse process of hydrogenation. Dehydrogenation reactions are conducted both on industrial and laboratory scales. Dehydrogenation converts saturated fats to unsaturated fats. Enzymes that catalyze dehydrogenation are called dehydrogenases.
How do you get dehydrogenation?
Dehydrogenation is the process by which hydrogen is removed from an organic compound to form a new chemical (e.g., to convert saturated into unsaturated compounds). It is used to produce aldehydes and ketones by the dehydrogenation of alcohols.
Is propene to propane oxidation?
Propene oxidation. To establish whether propane and propene oxidation result in the formation of the same oxygen-bearing products, propene oxidation was performed under the same conditions. The measurements show that initial propane conversion is considerably higher when compared to propene oxidation (Fig.
What is dehydrogenation reaction with example?
1 Dehydrogenation. Dehydrogenation is the removal of hydrogen from the parent molecule. For example, at 550°C (1025°F) n-butane (C4H10) loses hydrogen to produce butene-1 (CH3CH2CH=CH2) and butene-2 (CH3CH=CHCH3).
What is the difference between dehydration and dehydrogenation?
Dehydration is the reaction which involves removal or release of one or more molecules of water while the dehydrogenation is the reaction which involves the removal of one or more hydrogen molecules.
What type of reaction is hydration?
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne.
Does dehydration cause oxidation?
Dehydration can lead to an oxidative stress by increasing ROS production or by inactivating antioxidant enzymes or both. Traditionally, the impairment caused by increased ROS is thought to result from random damage to DNA, proteins and lipids.
What type of reaction is dehydration?
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction.
What is the process of dehydration?
Dehydration occurs when you use or lose more fluid than you take in, and your body doesn't have enough water and other fluids to carry out its normal functions. If you don't replace lost fluids, you will get dehydrated.
What mechanism is dehydration?
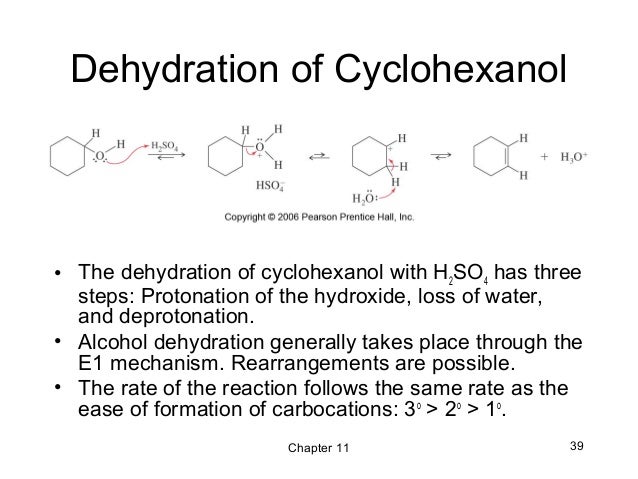
Primary alcohols dehydrate through the E2 mechanism. The hydroxyl oxygen donates two electrons to a proton from sulfuric acid (H2SO4), forming an alkyloxonium ion.
What is the temperature of oxidation of ethane?
When ethane and oxygen come into contact with a monolith at a temperature of about 573 K, rapid oxidation of ethane to H 2 O, CO, and CO 2 occurs on the surface of the platinum. The heat released by these reactions increases the temperature markedly. In a typical situation, the temperature increases to a level of 1123-1173 K at a distance through the monolith where only about one-fifth of the ethane has been converted. At these temperatures, the rates of homogeneous gas reactions become high enough for them to be responsible for virtually all of the remaining conversion of the ethane along the monolith. The production of ethylene and acetylene is attributed almost exclusively to reactions occurring in the gas phase. The heat required to sustain the endothermic dehydrogenation reactions yielding these products in the tail end of the reaction zone is supplied by exothermic gas-phase oxidation reactions that form additional H 2 O and CO.
What is chromium oxide catalyst?
400 nm) is a new attractive catalyst applicable for propane dehydrogenation to propylene with CO 2. The catalyst exhibits substantially higher activity than chromium oxide supported on ZSM-5 with larger crystal size (ca. 2 μm). This was due to the former catalyst having a much higher concentration of surface Cr 6+ species than the latter one, which accounts for its superior catalytic performance. In addition to the coke formation, the reduction of Cr 6+ to Cr 3+ is also responsible for the catalyst deactivation [133,169]. The oxidative dehydrogenation of propane to propylene was also investigated over a series of chromium-based catalysts supported on MFI zeolite materials. Structural characterization suggests that the boron present in the framework modified the nature of introduced Cr species. The steaming process resulted in auto-reduction of some Cr 6+ to Cr 3+ on Cr/H [B]MFI. A significantly enhanced stability was achieved over the steaming-treated catalyst, which was possibly attributed to the related Cr 3+ oxide species.
Is V-silicalite oxidative dehydrogenation?
In the oxidative dehydrogenation of lower alkanes, a V-containing-silicalite showed a lower activity but an higher selectivity towards alkenes [127,136a] and aromatics [116] compared to H-ZSM-5. In the oxidative dehydrogenation of propane, Zatorski et al. [ 136a] observed a very high selectivity to propene with either O 2 or N 2 O, using a catalyst with a very low vanadium content (see Table 6 ). The observed behaviour for V-silicalite and other modified silicalites was at least partially attributed to the presence of defect sites but no hypothesis was given on the reaction mechanism. On the basis of an accurate physical-chemical characterization of the same catalyst, Bellussi et al. [ 136 b] later suggested the following reasons for its remarkable selectivity:
How is dehydrogenation achieved?
Dehydrogenation can be achieved by direct removal of hydrogen from alkanes. Catalytic dehydrogenation of alkanes is an endothermic reaction, which occurs with an increase in the number of moles and can be represented by the expression
What is the selectivity of steam based dehydrogenation?
To my knowledge, the typical selectivity of the steam-based direct dehydrogenation system used predominantly by industry is very high, i.e., much more than 95%. However, inherently this process can tolerate lower selectivity, without much of an economic impact, because benzene and toluene are the by-products that can be recovered for sale/reuse. On the other hand, selectivity is critical for ODH. Lower selectivity will naturally result in products of combustion of ethylbenzene, and consequently wasted. For example, consider the data from Zhao et al. [392], they have presented a comprehensive comparison of as-produced and treated nanofibers for use as catalysts in the ODH of ethylbenzene to styrene. The optimum result (~60% conversion, 85% styrene selectivity, 12% CO 2 selectivity) is obtained by acid-treated herringbone GN. Though the edge sites are shown to have higher activity, this comparison only serves to show the relative performance of the different nanofibers. Let us take a closer look: Assuming the optimum result is identical to the numbers achieved by the incumbent process, and using data from Zhao, Fig. 7.10 A shows the mass balance with ODH and Fig. 7.10B shows the mass balance of direct dehydrogenation used by industry today. The loss of feed as CO 2 is significant in Fig. 7.10 A. In the real world, the selectivities are much higher than assumed here, and the numbers end up looking even less attractive. To be clear, Zhao et al. do not claim their work could be applied industrially to reduce costs of production of styrene. But the data should not be misconstrued as that consequence being an obvious reality. However, Xu et al. do claim ODH can replace direct dehydrogenation of ethyl benzene [309] due to the energy savings afforded by the exothermic ODH reaction. The conversion data presented does not seem to have been derived using the knowledge mentioned earlier in the document about the coke formation being a major contributor of catalytic activity. The commercial catalysts were run for only 6 hours, while coke formation, and consequently the catalytic activity of the “commercial” catalysts increases over time. The conversion rate of 4% for the commercial catalyst is unrealistically low under the operating conditions. The data is not sufficient to determine how long it took the alumina catalyst to achieve the equilibrium temperature of 547°C. The experimental section does not specify if the reactor and commercial catalyst were preheated or not, and what the exit temperature of the helium gas was. Based on T2 – T1 of the He, one could calculate the total heat transfer that occurred to the alumina catalyst per unit of time and determine the length of time it took to reach the reaction temperature. At 100% heat transfer efficiency, the catalyst would take 25 minutes to reach the reaction temperature. At 7200 per hour GHSV (calc.), this would be highly improbable. Without long derivations, it would be reasonable to assume that on the average the heat transfer efficiency would be closer to 10–30% as the differential temperature keeps getting smaller over time. At this rate, the catalyst itself would take approximately 2–4 hours to heat up to the reaction temperature. Therefore the effect of a relatively cooler catalyst surface temperature on the reactants is important to study and analyze.
What is ODH in chemistry?
Oxidative dehydrogenation (ODH) is a new alternate route to accomplish many dehydrogenation reactions. ODH is an exothermic process compared to the highly endothermic route currently utilized in industry as explained above. Ostensibly then, ODH has the potential to reduce costs. Some of the benefits include less coking, eliminating the high temperature furnace and obtaining higher yields of olefins. ODH can be applied to some very important industrial small chain alkane to olefin conversions. Ethyl benzene to styrene is a promising and potentially large volume application.
What are the drawbacks of paraffin dehydrogenation?
The huge number of papers devoted to the reaction of paraffin oxidative dehydrogenation is a demonstration of the current scientific and industrial interest for alternatives to catalytic and thermal dehydrogenation/cracking reactions which suffer from several drawbacks: i) thermodynamic limitations on paraffin conversion ; ii) side reactions such as thermal cracking ; iii) strongly endothermic reactions to which large amounts of heat must be supplied at temperatures above the reaction temperature; iv) formation of coke on the catalyst which requires frequent regeneration (1,6,34-36). With the goal of overcoming these limitations, research is proceeding along some directions, of which the following are the most likely to be implemented: i) optimization of current dehydrogenation technologies, to obtain more selective, stable and environmentally safe catalysts and lower investments and utility costs; ii) dehydrogenation coupled with hydrogen oxidation, to supply the heat of reaction inside the catalytic bed while avoiding overheating and to shift the equilibrium toward the desired products; iii) oxidative dehydrogenation, to overcome thermodynamic limitations, operate at low temperature with an exothermic reaction, and avoid frequent catalyst regeneration, and iv) membrane-assisted dehydrogenation, to obtain high conversion at low temperatures and to conduct the reactions and separations in the same equipment. In oxidehydrogenation the advantage gained from the use of a cheaper raw material and of an exothermic process has to deal with drawbacks such as i) the loss of valuable hydrogen (coproduced in dehydrogenation and in steam-cracking), ii) the difficulty in separation of CO from the paraffin (in the case of ethane oxidehydrogenation), and iii) the formation of traces of corrosive by-products. Moreover, while in the case of ethane the problem mainly concerns the low reactivity of the molecule (the selectivity to the olefin which can be achieved is high, due to the low ethylene reactivity and to the nature of the mechanism involved), in the case of oxidehydrogenation of propane and of n-butane to the corresponding olefins the selectivity problem is of main concern.
What temperature does cyclohexane dehydrogenate?
The oxidative dehydrogenation of cyclohexane to benzene has been reported to occur above 580 °C on different catalysts [1 - 4]. We have found that titania modification with molybdenum oxides resulted in a dramatic change of selectivity from CO 2 to benzene (up to 70%) at 35 °C under UV illumination in a gas-solid continuous photoreactor [ 5, 6 ]. More recently we reported the selective formation of benzene from cyclohexane on MoO x /TiO2 in a fluidized bed photocatalytic reactor [7]. In this work the performance of a two-dimensional fluidized bed photoreactor has been studied in comparison with an annular fixed bed reactor.
What is the temperature of oxidation of ethane?
When ethane and oxygen come into contact with a monolith at a temperature of about 573 K, rapid oxidation of ethane to H 2 O, CO, and CO 2 occurs on the surface of the platinum. The heat released by these reactions increases the temperature markedly. In a typical situation, the temperature increases to a level of 1123-1173 K at a distance through the monolith where only about one-fifth of the ethane has been converted. At these temperatures, the rates of homogeneous gas reactions become high enough for them to be responsible for virtually all of the remaining conversion of the ethane along the monolith. The production of ethylene and acetylene is attributed almost exclusively to reactions occurring in the gas phase. The heat required to sustain the endothermic dehydrogenation reactions yielding these products in the tail end of the reaction zone is supplied by exothermic gas-phase oxidation reactions that form additional H 2 O and CO.
Is NiO oxidative dehydrogenation active?
Previous work has demonstrated that NiO is highly active for ethane oxidative dehydrogenation. Activity has been observed at temperatures as low as 275°C. For catalysts prepared using a sol-gel technique, the ethylene reaches a maximum yield of 23% at 350°C. Ethylene selectivity is 47%. Above this temperature, selectivity declines precipitously.
What is the process of oxidation?
Oxidation of organic compounds generally involves the addition of O or the removal of two H from adjacent atoms. (Forming an alcohol from an alkyl group, forming a carbonyl group from an alcohol, or removing H from two adjacent carbons so they can form a pi bond)
Why is glucose oxidized?
For that oxidation of glucose via respiration plays an important role. Glucose is oxidized by every living cell to get the energy for their daily life process. Glucose can be oxidized either completely or partially.
How does dehydration work?
Dehydration removes H from one atom and OH from another in order to form a new bond between those two atoms, and produces water as an additional product.
What gives CO2 and H20 energy?
Complete oxidation of glucose gives the CO2 and H20 with energy.
What is the process of adding oxygen to a species?
Oxidation can be referred to as the addition of oxygen/release of hydrogen/loss of electrons by a specie.Oxidation usually increases the oxidation state of the species that’s being oxidized. See simple formation of water:
What happens to the products of electrolysis?
By virtue of their combined chemical energy, the products of an electrolytic process have a tendency to react spontaneously with one another, reproducing the substances that were reactants and were therefore consumed during the electrolysis. If this reverse reaction is allowed to occur under proper conditions, a large proportion of the electrical energy used in the electrolysis may be regenerated. This possibility is made use of in accumulators or storage cells, sets of which are known as storage batteries. The charging of an accumulator is a process of electrolysis; a chemical change is produced by the electric current passing through it. In the discharge of the cell, the reverse chemical change occurs, the accumulator acting as a cell that produces an electric current.”
What is the process whereby electrical energy is converted directly into chemical energy?
A process whereby electrical energy is converted directly into chemical energy is one of electrolysis; i.e., an electrolytic process.
What is dehydrogenation reaction?
Dehydrogenation reactions in the presence of oxygen are conducted on silver catalysis to transform alcohols into the corresponding aldehydes. The reaction types can be extended to prepare fine chemicals thus dec-9-en-1-ol was on silver catalysis with good yields. In contrast, dehydrogenation reactions can be conducted in the absence of oxygen on platinum or palladium catalysts to aromatize substituted cyclohexyl or cyclohexenyl compounds. Thus, in the field of fine chemistry p-cymene was obtained by dehydrogenation of limestone with a 67% yield on active carbon supported Pd catalysts.
Why is dehydrogenation important in petroleum?
Dehydrogenation is one of the most important processes in the chemistry of petroleum because it turns the starting inert alkanes into olefins and aromatic compounds, starting points towards other functional groups.
What is Dehydration of Alcohols?
Alcohol upon reaction with protic acids tends to lose a molecule of water to form alkenes. These reactions are known as dehydrogenation or dehydration of alcohols.
What was the purpose of the dehydrogenation of butane?
During the Second World War, catalytic dehydrogenation of butane over a catalyst for chromium – alumina was practiced to generate butenes that were dimerized to octenes and hydrogenated to octans to yield high octane aviation fuels.
What is the role of catalytic dehydrogenation in the development of olefin light?
During the Second World War, catalytic dehydrogenation of butane over a catalyst for chromium – alumina was practiced to generate butenes that were dimerized to octenes and hydrogenated to octans to yield high octane aviation fuels.
Which step is the slowest in the mechanism of dehydration of an alcohol?
In this step, the C-O bond breaks generating a carbocation. This step is the slowest step in the mechanism of dehydration of an alcohol. Hence, the formation of the carbocation is considered as the rate-determining step.
Which mechanism does dehydration follow?
Dehydration of alcohols can follow E1 or E2 mechanism. For primary alcohols, the elimination reaction follows E2 mechanism while for secondary and tertiary alcohol elimination reaction follows E1 mechanism.
What is catalytic dehydrogenation?
Catalytic dehydrogenation is a critical and growing technology for the production of olefins, especially for propylene production. This paper will give an overview of advances in the catalysis science and technology for production of olefins by catalytic dehydrogenation, including the concomitant removal of H2 by selective oxidation. For light paraffin dehydrogenation, UOP has licensed the Oleflex™ process widely for production of polymer-grade propylene as well as isobutylene with over 12 million metric tons of capacity announced. Today there are nine UOP C3 Oleflex™ units in operation accounting for 55 % of the installed world-wide propylene production capacity from propane dehydrogenation technology. The heart of the process is a noble metal multi-metallic catalyst and the continuous catalyst regeneration (CCR) process. The coupling of catalytic dehydrogenation with selective oxidation of hydrogen allows one to design a process, which greatly improves equilibrium conversions while maintaining very high selectivity to olefin. The Lummus/UOP SMART™ SM process (Styrene Monomer Advanced Reheat Technology) allows 30–70 % capacity expansion, achieves a higher per-pass ethylbenzene conversion, and provides the most cost-effective revamp for higher capacity. Styrene Monomer Advanced Reheat Technology (SMART™) uses an oxidation catalyst and novel reactor internals to allow oxidative reheating between dehydrogenation stages. In the case of selective oxidation catalysts containing dispersed metal active sites, the role of diffusion and pore architecture is as important as the active metal sites.
What is the dehydrogenation of unactivated positions?
The dehydrogenation at saturated, unactivated positions in simple as well as complex organic compounds is a considerable challenge that requires rather unique methods that are able to overcome the thermodynamic limitations while at the same time ensuring a chemoselective progress of the reaction. Two major concepts are discussed within this chapter to create unsaturation at unactivated positions: the generation of radical intermediates and the activation of C–H bonds by metals. In addition, a selection of dehydroaromatization reactions is shown to be of immense importance for the formation of aromatic carbo- and heterocycles.
What temperature does methylcyclohexane convert to?
The selective, catalytic conversion of methylcyclohexane into methylenecyclohexane at 100°C, and of n-hexene into 1-hexene at 45°C , has been effected using bis (triisapropylphosphine)iridium pentahydride and an olefin (neohexene) as a hydrogen acceptor; with this system, approximate relative reactivities of C-H bonds in saturated hydrocarbons are: sec-alkyl-H, 1; iso-alkyl-H, 8; n-alkyl-H, > 60.
What is the temperature of dehydrogenation of cycloöctane?
Homogeneous catalytic dehydrogenation of cycloöctane into cycloöctene has been effected by means of a variety of iridium and ruthenium polyhydrides, at 150°C, in the presence of an olefin as the hydrogen acceptor; best results (45–70 catalytic turnovers) have been achieved with (iPr3P)2IrH5, [ (-F-C6H4)3P]2IrH5 and [ (-F-C6H4)3P]3RuH4.
How are n-alkenes obtained?
n-Alkenes and dihydrogen were obtained from n-alkanes by photocatalysis using carbonyl (chloro)phosphine–rhodium complexes; the rate of alkane dehydrogenation was the same as that of propan-2-ol dehydrogenation under the same photocatalytic reaction conditions.
What is a Tandem dehydrogenation-olefin-metathesis catalyst?
Tandem dehydrogenation-olefin-metathesis catalyst systems, comprising a pincer-ligated iridium-based alkane dehydrogenation catalyst and a molybdenum-based olefin-metathesis catalyst, are reported to effect the metathesis-cyclooligomerization of cyclooctane and cyclodecane to give cycloalkanes with various carbon numbers, predominantly multiples of the substrate carbon number, and polymers.
Which complexes catalyze the transfer dehydrogenation of alkenes under an atmosphere of hydrogen?
Arsine complexes of rhodium (I) efficiently catalyze the transfer dehydrogenation of alkenes under an atmosphere of hydrogen, while at the same time producing decreased levels of ‘direct hydrogenated’ sacrificaial olefin relative to that derived from similar phosphine-based catalysts.
Abstract
A great suCCess: The oxidative dehydrogenation (ODH) of ethane is a process that enables the production of ethene with high selectivities. Different catalysts and reactor configurations have been developed for this process.
Abstract
The increasing demand for light olefins and the changing nature of basic feedstock has stimulated substantial research activity into the development of new process routes. Steam cracking remains the most industrially relevant pathway, but other routes for light-olefin production have emerged.
