Dissolution of ammonium chloridein water is an endothermic reaction, yet it is a spontaneous process. This is due to the fact that : A. ΔH is +ve, ΔS is -ve. Thereof, why dissolution of ammonium chloride in water is endothermic?
Is dissolution of ammonium chloride endothermic or exothermic?
The dissolution of ammonium chloride in water is endothermic process. What is the effect of temperature on its solubility ? The dissolution of ammonium chloride in water is endothermic process. What is the effect of temperature on its solubility ?
What is the purpose of dissolving ammonium chloride?
The dissolution of ammonium chloride is used to cool a container of water placed in the solution. It’s an endothermic process. What happens during dissolving? A solvent is the substance that does the dissolving – it dissolves the solute.
What happens when H2O is added to ammonium chloride?
When water (H2O) is added to ammonium chloride (NH4Cl), the negative part of our dipole NH4Cl i.e. the electron density on Cl- separates H+ from water and it dissociates to its traditional H+ and OH- ions for which it needs some dissociation energy as H-O bond is strong.
Is dissolving of NaCl in water endothermic or exothermic?
Dissolution of NaCl in waterDissolution of sodium chloride in water is endothermic. Is ice melting endothermic or exothermic? Contents Is ice melting endothermic or exothermic? Is cooking an egg endothermic or exothermic? Is nh4cl exothermic or endothermic? What happens during dissolving? What are the 3 steps in the dissolving process?
See more

Is dissolving ammonium salt in water endothermic?
Ammonium nitrate dissolving in solution is an endothermic reaction.
Is ammonium chloride dissolving in water exothermic or endothermic?
endothermic processThe dissolution of ammonium chloride in water is an endothermic process.
What happens when you dissolve ammonium chloride in water?
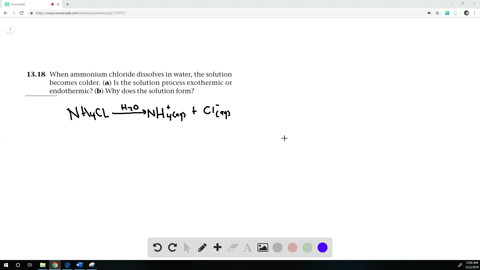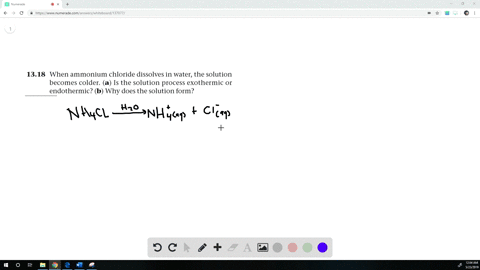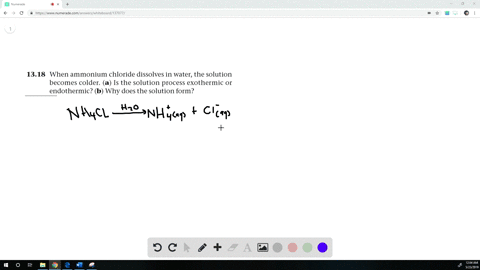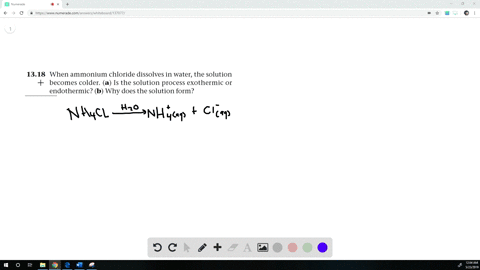
When ammonium chloride (NH4Cl) is dissolved in water H2O, it will dissociate (dissolve) into NH4+ and Cl− ions.
Why is the dissolving of ammonium chloride endothermic?
The value of the enthalpy change i.e. ΔH for the dissolution reaction of ammonium chloride is +15.1 kJ mol−1. A positive value of ΔH indicates that the dissolution reaction of ammonium chloride is an endothermic process. In an endothermic process, heat is absorbed. Thus, ΔH is positive.
Is dissolving ammonium chloride in water a physical or chemical change?
1 Answer. No, ammonium chloride in water is not a physical reaction. It is a chemical reaction.
When ammonium chloride is dissolved in water the solution becomes cold?
When Ammonium chloride is dissolved in water, it undergoes an endothermic reaction. Thus, the solution becomes cooler. Hence, the change is endothermic.
Is dissolving salt in water endothermic or exothermic?
endothermicThe enthalpy of solution of NaCl in water (that is energy change associated with the dissolution of sodium chloride crystals in water) at standard condition is very slightly positive, it is an endothermic process.
What type of reaction is NH4Cl h2o?
0:331:18Equation for NH4Cl + H2O (Ammonium chloride + Water)YouTubeStart of suggested clipEnd of suggested clipSo we'll have this ammonium ion this nh4. Plus. And then the chloride ion. Because these two ionsMoreSo we'll have this ammonium ion this nh4. Plus. And then the chloride ion. Because these two ions are in water they're dissolved in water we can write an aq after each one which means aqueous.
When NH4Cl is added to water then heat is?
When solid ammonium chloride, NH4Cl, is added to water, the temperature of the solution decreases. Which statement best describes this observation? The reaction is exothermic NH4Cl(s) does not dissolve in water. Heat is released from the system, so it feels cooler.
Is the dissolution of cacl2 exothermic or endothermic?
exothermic reactionWhen dissolved in water, solid calcium chloride releases heat in an exothermic reaction.
What happens when ammonium chloride is heated?
Ammonium chloride appears to sublime upon heating but actually decomposes into ammonia and hydrogen chloride gas: NH4Cl → NH3 + HCl.
How can you conclude that dissolution of ammonium chloride in water is an exothermic change?
Answer: Dissolution of ammonium chloride in water is endothermic as the solution becomes colder. It means that it absorbs heat which means it is an endothermic reaction.
Is dissolving NaCl endothermic or exothermic?
endothermicThe enthalpy of solution of NaCl in water (that is energy change associated with the dissolution of sodium chloride crystals in water) at standard condition is very slightly positive, it is an endothermic process.
Is dissolving cacl2 in water exothermic or endothermic?
exothermic reaction3. When dissolved in water, solid calcium chloride releases heat in an exothermic reaction.
Is the decomposition of ammonium chloride exothermic?
The decomposition of ammonium chloride into ammonia and hydrogen chloride is endothermic (heat absorbed or heat taken in from the surroundings - the hot test tube).
How can you conclude that dissolution of ammonium chloride in water is an exothermic change?
Answer: Dissolution of ammonium chloride in water is endothermic as the solution becomes colder. It means that it absorbs heat which means it is an endothermic reaction.
Does NH4Cl increase solubility?
Since dissolution of NH4Cl in water is endothermic process, its solubility increases with rise in temperature (i.e., Le-Chatelier process).
Is ammonium chloride dissolved in water?
Dissolution of ammonium chloride in water is an endothermic reaction, yet it is a spontaneous process. This is due to the fact that
Is ammonium chloride endothermic or endothermic?
The dissolution of ammonium chloride in water is an endothermic process but still it dissolves in water readily. Why ?
Why is ammonium chloride acidic?
Acidic. It is because hydrolysis of ammonium chloride gives ammonium hydroxide and hydrogen chloride. Hydrogen chloride being stronger, dissociated to give hydrogen ions and makes resulting solution acidic.
How to separate nacl from water?
In my point of view distillation is the simplest process to separate nacl from water because boiling point of salt is higher than water, so by distillation process water evaporates easily then salt resides on the dish as solid. And the vapours of water will be condensed by the condenser and collected on another beaker.
How to explain salt hydrolysis?
That is, first dissociating salt and water into their constituent ions then interacting the ions furnished by the salt and water. And finally writing the sum, which shows there is hydrogen ions in resultant solution indicating th
What is sodium chloride made of?
Sodium chloride or table salt is made up of positively ( Na+)nd negatively charged ions (Cl-) nd they are held together by the strong electrostatic force of attraction (in the solid state).
What is the energy behind the dissolution of ions?
There is an energy behind the dissolution of the ions..nd that energy is called Lattice energy which keeps the oppositely charged ions away from each other. So, this energy is added to the solution .
Which ion hydrolyzes according to the equilibrium?
The ammonium ion hydrolyzes according to the equilibrium (read until the end, you will be surprised)
Is hydration energy exothermic or endothermic?
If the hydration energy is greater than that of lattice energy then the solution is exothermic, whereas the reverse is true for endothermic reaction.
