
Which is better for the garden lime or gypsum?
Gypsum therefore improves soil conditions much more rapidly than lime and will affect soil conditions to a greater depth than lime will. Gypsum will supply calcium to deeper depths than lime. This will improve subsoil conditions, and allow for greater root growth (better nutrient and water efficiency).
What are the benefits of gypsum?
Benefits of Gypsum. Gypsum is an excellent source of Calcium and Sulfur (in the form of sulfate) for the soil. Calcium performs a variety of functions through natural chemical reactions to aid in better soil structure. One of these functions is to improve aggregation, which allows for better water movement and root growth.
Is lime stone and gypsum used in cement?
concrete is portland cement- the fine gray powder that binds sand and gravel into concrete's rock-like mass. The mining, production and uses of cement, clay and shale are Cement is made from gypsum, shale or clay, and limestone. "cement" is most often used to refer to Portland cement. Portland cement, when
Does lime soften clay soil?
One way of improving the texture of a clay soil is to add lime. This raises the pH of acid clay soils, making them more alkaline and in doing so it encourages clay particles to stick together in small clumps. This results in larger particles and makes the soil more friable and easier to work.
See more
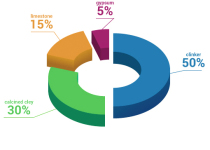
Can you use gypsum instead of lime?
Lime and gypsum both work to add calcium to your soil. Gypsum can also remove sodium and add sulfur to soil, but it can't balance pH levels like lime can.
Is gypsum is made from lime?
Limestone and gypsum are minerals that form from calcium salts; limestone contains calcium carbonate while gypsum contains CaSO4·2H2O. However, their properties and usages are different from each other.
Whats better gypsum or lime?
Gypsum therefore improves soil conditions much more rapidly than lime and will affect soil conditions to a greater depth than lime will. Gypsum will supply calcium to deeper depths than lime. This will improve subsoil conditions, and allow for greater root growth (better nutrient and water efficiency).
Can you put too much gypsum on your garden?
Can You Apply Too Much Gypsum to Your Soil? Yes, you can. Adding too much gypsum to the soil can lead to beneficial elements such as aluminum, magnesium, iron, and manganese getting eliminated. The lack of these nutrients can hinder the growth of plants.
What does gypsum do for your soil?
Gypsum helps soil better absorb water and reduces erosion. It also cuts down on phosphorus movement from soils to lakes and streams and improves the quality of various fruits and vegetables, among other benefits.”
Can I mix lime and gypsum?
Gypsum can be spread with lime and litter spreaders. Gypsum is not acid soluble and will not change the soil pH. It helps to shift the Ca and Mg levels in soil and offers a readily available form of sulfate sulfur, a valuable secondary nutrient that benefits the soil and crop.
When should you apply gypsum?
Gypsum does not change the pH of your soil, so you can use it around acid-loving plants such as rhododendrons, azaleas and blueberries to provide extra calcium. Although best applied in the fall, lime can be applied at any time of the year.
Should I put gypsum in my garden?
Adding gypsum to your garden is a great way to ensure your plants have sufficient sulfur. Unlike elemental sulfur that is unavailable to plants until it is oxidized by soil bacteria, the sulfate in gypsum is ready for plant absorption immediately.
What is gypsum and lime?
Lime, also known as agricultural limestone, neutralizes soil acidity and provided calcium and magnesium available for plant uptake. Gypsum is a calcium sulfate containing product that provides both calcium and sulfate to the soil system. No change in pH can be expected from a gypsum application.
What is lime used for?
Lime is the versatile mineral. Various forms of lime are used in environmental, metallurgical, construction, and chemical/industrial applications, and more. The fastest growing use of lime is in environmental applications, where lime is used to comply with air, drinking water, wastewater, and solid waste regulations.
What is the difference between lime plaster and gypsum plaster?
Lime sets slowly by absorbing carbon dioxide from the air, whereas gypsum plaster sets rapidly by crystallising (even fully hydrated gypsum plaster sets within about a day). Also, as a lime plaster dries it shrinks slightly, while a gypsum plaster expands slightly as it sets.
What is the formula for gypsum?
Gypsum is the name given to a mineral categorized as calcium sulfate mineral, and its chemical formula is calcium sulfate dihydrate, CaSO4⋅ 2H2O.
Gypsum and Lime Both Improve Soil Conditions but They Have Vast Differences
Ag lime and gypsum are excellent soil amendments that can be used separately, together, or in a rotation to improve soil conditions. However, under...
What Is Agricultural Lime?
Ag lime is an acid-soluble material that is applied to cropland to raise the pH of acidic soils. It comes in many forms including calcium carbonate...
Proper Levels of Calcium and Magnesium
The Cation Exchange Capacity (CEC) in soil measures the nutrient holding capacity, or the number of exchange sites in a given volume of soil that a...
Choosing Lime, Gypsum Or Both
When choosing to use lime, gypsum, or both products, start with accurate soil test results, including the soil pH, CEC, organic matter and the base...
What is Gypsum?
Gypsum is calcium sulfate dihydrate (CaSO 4 2H 2 0). Flue gas desulfurization (FGD) gypsum, such as GYPSOIL ® brand gypsum, is a co-product material derived from the scrubbing of flue gas emissions in coal-burning power plants. Gypsum can be spread with lime and litter spreaders. Gypsum is not acid soluble and will not change the soil pH. It helps to shift the Ca and Mg levels in soil and offers a readily available form of sulfate sulfur, a valuable secondary nutrient that benefits the soil and crop. The sulfate in gypsum binds with excess Mg in the soil to form soluble Epsom salt, which moves lower into the soil profile. This Mg is replaced by Ca, improving water holding capacity, root development and soil quality.2
What is Ag lime?
Ag lime is an acid-soluble material that is applied to cropland to raise the pH of acidic soils. It comes in many forms including calcium carbonate (CaCO 3 ), magnesium carbonate (MgCO 3) and others. It can be applied in a single pass with a lime spreader. Acidic soils trigger a chemical reaction allowing carbonate molecules to strip H+ molecules ...
What is the cheapest form of lime?
It is important to consider the Ca and Mg levels on the soil exchange sites before choosing a lime source. Magnesium carbonate, called dolomitic lime, is often the cheapest form of lime.
Can gypsum be spread with lime?
Gypsum can be spread with lime and litter spreaders. Gypsum is not acid soluble and will not change the soil pH. It helps to shift the Ca and Mg levels in soil and offers a readily available form of sulfate sulfur, a valuable secondary nutrient that benefits the soil and crop. The sulfate in gypsum binds with excess Mg in ...
Is gypsum a soil amendment?
Gypsum and Lime Both Improve Soil Conditions But They Have Vast Differences. Ag lime and gypsum are excellent soil amendments that can be used separately, together, or in a rotation to improve soil conditions. However, understanding the differences between lime and gypsum, and how they impact soil chemistry, is important when choosing ...
Does gypsum bind with Epsom salt?
The sulfate in gypsum binds with excess Mg in the soil to form soluble Epsom salt, which moves lower into the soil profile. This Mg is replaced by Ca, improving water holding capacity, root development and soil quality.2.
Do all soils have the same CEC?
Not all soils have the same CEC, for example, soils with more clay and organic matter have a greater holding capacity than sandy soils with less organic matter. Amendment rates vary by soil CEC. Base Saturation: The base saturation is the percentage of exchange sites occupied by positively charged atoms and molecules such as Ca++, Mg++, H+, K+, ...
What is gypsum made of?
Gypsum is hydrated calcium sulfate, CaSO4·2H2O, that comes in the form of flattened crystals. It is mined from geologic deposits and produced artificially as a by-product of devices used to control pollution in coal-fired power plants. It is also used to make chalk, plaster of Paris and drywall for building construction.
What are the two types of lime?
Types of Lime. Two kinds of lime, CaO , are ordinarily used to raise the pH of soil. Calcitic limestone is largely calcium carbonate, CaCO2. Dolomitic limestone, made from rocks containing magnesium carbonates and calcium, must be at least 6 percent magnesium.
Why is gypsum used in soil amendments?
Both gypsum and lime are used as soil amendments. The usefulness of comparing them is limited because their function is different . Gypsum is added to clay and heavy soils to help them drain and improve their tilth, or ability to be tilled, and to remove salt from saline soils. Lime is added to improve the pH of acidic soils.
What is lime used for?
Both gypsum and lime are used as soil amendments.
Why is gypsum added to clay soil?
Gypsum is added to heavy clay soil to improve its ability to drain and to improve its texture for easier cultivation.
What is the pH of lime?
Soil pH measures the potential of the hydrogen ion in water; an ion is an atom or molecule with a positive or negative charge. The pH scale of 1 to 14 is a logarithm ; a soil with a pH of 5 has 10 times as much active hydrogen as pH 6 soil. A soil with pH 7 is neutral; soils below 7 are acidic; soils above 7 are alkaline.
Is gypsum a logarithm?
The pH scale of 1 to 14 is a logarithm; a soil with a pH of 5 has 10 times as much active hydrogen as pH 6 soil. A soil with pH 7 is neutral; soils below 7 are acidic; soils above 7 are alkaline. Gypsum is added to heavy clay soil to improve its ability to drain and to improve its texture for easier cultivation.
What is gypsum made of?
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4·2H2O. It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard/sidewalk chalk, and drywall. A massive fine-grained white or lightly tinted variety of gypsum, called alabaster, has been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England.
What color is lime?
The color of the lime {1}, a yellowish-green.
What is a white alkaline substance?
a white alkaline substance consisting of calcium hydroxide, made by adding water to quicklime and used in traditional building methods to make plaster, mortar, and limewash.
What is lime used for?
treat (soil or water) with lime to reduce acidity and improve fertility or oxygen levels
What is selenite made of?
A mineral consisting of the hydrous sulphate of lime (calcium). When calcined, it forms plaster of Paris. Selenite is a transparent, crystalline variety; alabaster, a fine, white, massive variety.
What is plaster of Paris made of?
A mineral consisting of hydrated calcium sulphate. When calcined, it forms plaster of Paris.
What is a pale timber tree?
a deciduous tree with heart-shaped leaves and fragrant yellowish blossom, native to north temperate regions. The pale timber is used for carving and inexpensive furniture.
What is FGD gypsum?
FGD gypsum (which stands for flue gas desulfurization) is produced at coal-burning power plants in the process of removing sulfur from air emissions, and is approved for land-application in Kansas.
Does gypsum affect soil pH?
Unlike lime, it does not affect soil pH — elemental sulfur is the correct form for reducing pH. Gypsum is about 200 times more soluble than lime and is naturally found in the soil profiles of the more arid parts of the state.
Is gypsum a form of alabaster?
Gypsum, on the other hand, is CaSO4.H2O. It can occur in many forms — selenite is the crystal form, while alabaster is powdery. Most commonly used in Kansas to remediate sodic soils, a ton of gypsum contains about 320 pounds of sulfur. Unlike lime, it does not affect soil pH — elemental sulfur is the correct form for reducing pH.
What is the color of a lime tree?
A light, somewhat yellowish , green colour associated with the fruits of a lime tree.
What is the West Indies lime?
to treat with calcium hydroxide or calcium oxide (lime) or lime can be (west indies) to hang out/socialize in an informal, relaxed environment, especially with friends, for example at a party or on the beach.
What is the product of burning of chalk or limestone?
Lime , which is the product of the burning of chalk or limestone, might be bought ready burnt, or it could be burnt in kilns specially constructed in the neighbourhood of the building operations.
What is plaster of Paris made of?
A mineral consisting of the hydrated calcium sulphate. When calcined, it forms plaster of Paris.
Is citrus a tropical plant?
* Both this and the citrus are trees with fragrant flowers, but this is more temperate and the citrus is more tropical and subtropical. Outside of Europe and adjoining parts of Asia, the citrus sense is much more common
Is gypsum a mineral?
is that gypsum is a mineral consisting of the hydrated calcium sulphate when calcined, it forms plaster of paris while lime is (chemistry) a general term for inorganic materials containing calcium, usually calcium oxide or calcium hydroxide; quicklime or lime can be a deciduous tree of the genus tilia , especially ; the linden tree, or its wood or lime can be any of several green citrus fruit, somewhat smaller and sharper-tasting than a lemon or lime can be (anime) a fan fiction story that stops short of full, explicit descriptions of sexual activity, with the intimacy left to the reader's imagination.
Gypsum vs Lime
The main difference between gypsum and lime is in the component that is added to calcium for making it. Gypsum is the sulphate salt of Calcium and is used in building, and as a hardening component whereas Lime is the carbonate salt of Calcium and is used in chalks and other such substances.
What is Gypsum?
Gypsum is commonly a Calcium salt and contains sulphate and is known as hydrated calcium sulphate due to the presence of two molecules of water. The chemical formula of gypsum is CaSO4.2H2O. The formation of gypsum is due to the action of oxygen on the rocks.
What is Lime?
Lime is yet another salt of Calcium that has a different acidic group attached to it. The group that is attached is Carbonate and the chemical name of lime is Calcium Carbonate. The chemical formula is CaCO3. The formation of lime is due to the sedimentation process.
Main Differences Between Gypsum and Lime
The hydrated calcium sulphate or gypsum has the chemical formula as CaSO4 whereas the sedimented rock at the bottom of the ocean formed due to dead marine life is known as Lime.
Conclusion
Gypsum and lime both are very useful salts present in nature and their process of making is different. Gypsum is the sulphate whereas lime is the carbonate salt of Calcium.
What is the difference between lime and gypsum?
Lime is a carbonate, oxide or hydroxide of calcium. It is used to increase soil pH and provide calcium ions in the soil. Gypsum is calcium sulphate. It is also used to provide calcium ions in the soil, but does not have the effect of increasing soil pH.
Why are lime and gypsum grouped?
Lime and gypsum are broadly grouped as they are calcium-containing minerals which are used for soil amendment on agricultural soils.
What is a hydrated lime?
Hydrated lime. Hydrated lime, also known as slaked lime, is calcium hydroxide, as opposed to calcitic and dolomitic lime. It has a higher neutralising value than calciltic lime, but there are some challenges associated with using it.
What is the difference between calcitic lime and dolomitic lime?
to neutralise soil acidity, and to add calcium ions to the soil. There is one significant difference though. Dolomitic lime contains magnesium, whereas calcitic lime does not. This is an important distinction, as certain soils require additional magnesium, and other soils would be harmed through the addition of magnesium. It is important that the correct lime is used for the correct context.
How effective is lime?
There are two things that need to be considered in understanding the effectiveness of a specific lime source: 1) neutralising value and 2) fineness. The neutralising value gives an indication of how effective the lime will be at neutralising soil acidity, i.e. increasing soil pH. Ideally the neutralising value of lime should be greater than 95. The fineness gives an idea of the distribution of particle sizes of the lime. The finer the particles, and the greater the proportion of finer particles, the more effective the lime will be.
What is quick lime?
Quick lime. You may also come across quick (also called burnt) lime. This is calcium oxide. It is more concentrated than calcitic lime, but is unpleasant to handle, so is very seldom used in agriculture.
How much more effective is granulated lime?
A note on granulated lime. As wonderful as this sounds, it usually comes with a hefty price tag, often costing about ten times more than normal lime. This is probably why it is also often quoted as being ten times more effective, and therefore you would only need to use a tenth of the recommended rate of normal lime.
Why do you put lime and gypsum together?
So why would we want to apply gypsum and lime together? I will outline several reasons. First lime is insoluble in water so it is relatively immobile in the soil. Contrast that with gypsum which is water soluble and has much greater mobility in the soil.
How much lime/gypsum per acre?
A 50:50 lime/gypsum mix is most often used in our area. Typically, the rates of each product are 1000 or 2000 pounds per acre.
What is the most reactive lime?
The most reactive lime is the dust so you will not realize the benefits of this lime if it lands on your neighbor’s field. By applying gypsum with the lime you can apply a very high quality lime source and control the dustiness of the lime. The application will be dust free.
Does gypsum react with aluminum?
If aluminum toxicity is an issue due to acid subsoils, the gypsum will also react with the aluminum to offset its effect. As a result, root depth will be greater and nutrient availability will be improved. Lime can initially cause a cementing of the soil at the surface decreasing water infiltration. By applying gypsum with the lime you will negate ...
Is gypsum good for low pH soil?
Another misunderstanding is that gypsum is for high pH soils and lime is for low pH soils. In actuality, gypsum can be applied and has benefits in both high pH soil and low pH soils. Gypsum and lime applied together can actually have synergistic effects.
Can you mix lime and gypsum?
Applying Gypsum and Lime Together. A misunderstanding about lime and gypsum is that if you mix them or apply them together they will be antagonistic or “fight” one another. Another misunderstanding is that gypsum is for high pH soils and lime is for low pH soils. In actuality, gypsum can be applied and has benefits in both high pH soil ...
Does lime affect pH?
Lime will have a much more pronounced effect on soil pH than gypsum will, however, the pH change will be near the surface where the lime is placed. Lime is often times dusty and can become air borne when applied.
