Is H+ the strongest acid?
Hydroiodic acid (sometimes referred to as hydriodic acid) is an extremely acidic solution of hydrogen iodide and water. This compound is known to be the second strongest acid of hydrogen and a halogen (the strongest being hydrostatic acid). Hydroiodic acid is a widely used chemical reagent.
Is H+ a base or acid?
Similarly, is H+ an acid or a base? If one of those ions is H +, the solution is acidic. The strong acid hydrogen chloride (HCl) is one example. If one of the ions is OH-, the solution is basic. An example of a strong base is sodium hydroxide (NaOH). Beside above, how does H+ affect pH?
Is H+ the same as an electron?
The hydrogen atom consists of a proton, which forms the nucleus, and an electron which is found outside the nucleus. A hydrogen ion, H+, is just the proton, with a positive charge. To have a H+ ion the electron must have been removed. An electron, e^-, is a fundamental particle also found in the hydrogen atom, but not in the nucleus.
Is H+ an acidic radical?
What is meant by acidic radical? Acid radical is the ion formed after the removal of Hydrogen ion (H+) from an acid. Example: When H2SO4 loses H+ ion, it forms HSO4− which is an acid radical. The ion formed after the removal of hydroxide ion (OH−) from a base is known as basic radical.

Is acid OH or H?
When the number of hydrogen ions (H+)equals the number of hydroxide ions (OH-), a solution is said to be neutral. Acids increase the number of hydrogen ions (H+) in a solution (there are more hydrogen ions than hydroxide ions) and the resulting solution is said to be acidic.
What kind of acid is H?
Hydrochloric acid [H+(aq) Cl−(aq) or H3O+ Cl−], also known as muriatic acid, is an aqueous solution of hydrogen chloride (chemical formula: HCl). It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid.
Does H stand for acid?
pH stands for "power of hydrogen." The "H" is capitalized because it is the hydrogen element symbol. pH is a measure of how acidic or basic an aqueous solution is. It is calculated as the negative logarithm of hydrogen ion concentration.
What are examples of an acid?
Examples of acids include the inorganic substances known as the mineral acids—sulfuric, nitric, hydrochloric, and phosphoric acids—and the organic compounds belonging to the carboxylic acid, sulfonic acid, and phenol groups.
What are common acids?
15.2: Common Acids and Their UsesHydrochloric Acid.Sulfuric Acid.Nitric Acid.Carbonic Acid.Formic Acid.Acetic Acid.Citric Acid.Acetylsalicylic Acid.More items...•
Is H a strong base?
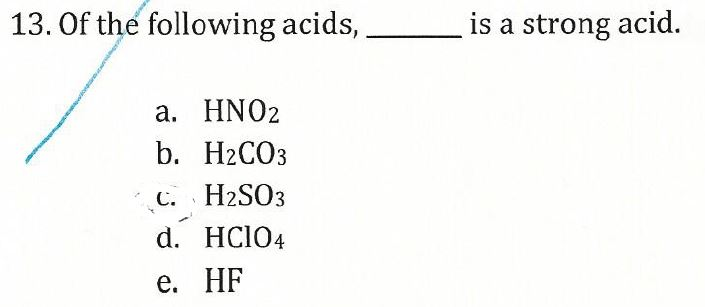
Water is the base that reacts with the acid HA, A− is the conjugate base of the acid HA, and the hydronium ion is the conjugate acid of water. By definition, a strong acid yields 100% of H3O+ and A− when the acid ionizes in water....Strong Acids.Strong AcidsStrong Basessulfuric acid (H2SO4)barium hydroxide (Ba(OH)2)5 more rows•May 8, 2021
What is the H in pH?
The H in pH stands for hydrogen. But, there is some controversy as to what the p represents. The Carlsberg Foundation itself says pH means "power of hydrogen". However, German chemists claim it stands for Potenz (also meaning power), whereas the French say it is their word for power, puissance.
Why are H+ ions acidic?
One water molecule gains a hydrogen and therefore takes on a positive charge, while the other water molecule loses a hydrogen atom and therefore becomes negatively charged. H 3O + is called a hydronium ion, and it makes things acidic. OH - is called a hydroxyl ion and it makes things basic.
What is an acid?
An acid is any compound that yields hydrogen ions (H+) or hydronium ions. Page 1. 1. An acid is a solution with more H+ ions than OH- ions. An acid is any compound that yields hydrogen ions (H+) or hydronium ions (H3O+) when dissolved in water. Does more H+ mean higher pH?
What happens to the pH scale as the pH increases?
The logarithmic scale of pH means that as pH increases, the H+ concentration will decrease by a power of 10. The higher the H+ concentration, the lower the pH, and the higher the OH- concentration, the higher the pH.
Is H+ a Lewis acid?
But yes, theoretically speaking H+ is a Brønsted–Lowry acid as the definition of such is to donate a proton and it donates itself to water in the case. It's also a Lewis acid as by donating itself it accepts electrons in its 1s emptry orbital. This whole paragraph relates to the solvent definition of acids and bases. Click to see full answer.
Can you have H+ by itself?
Well, you can never have H+ by itself. In aqueous media it's always associated with the lone pairs of water molecules and exists as H3O+. But yes, theoretically speaking H+ is a Brønsted–Lowry acid as the definition of such is to donate a proton and it donates itself to water in the case.
Is H3O+ an acid?
But be aware that in water, you never have H+, it's always H3O+ which is the strongest acid that can exist in water solutions, all acids are limited by the strength of H3O+ when dissolved in water. Therefore, a species is an acid in water if it generates H3O+.
Definition of H acid
You must — there are over 200,000 words in our free online dictionary, but you are looking for one that’s only in the Merriam-Webster Unabridged Dictionary.
Love words?
You must — there are over 200,000 words in our free online dictionary, but you are looking for one that’s only in the Merriam-Webster Unabridged Dictionary.
Where does hyaluronic acid come from?
Hyaluronic acid is derived from hyalos (Greek for vitreous, meaning ‘glass-like’) and uronic acid because it was first isolated from the vitreous humour and possesses a high uronic acid content. The term hyaluronate refers to the conjugate base of hyaluronic acid. Since the molecule typically exists in vivo in its polyanionic form, it is most commonly referred to as hyaluronan .
How much hyaluronic acid is in the body?
The average 70 kg (150 lb) person has roughly 15 grams of hyaluronan in the body, one-third of which is turned over (i.e., degraded and synthesized) per day. Hyaluronic acid is also a component of the group A streptococcal extracellular capsule, and is believed to play a role in virulence.
What is the name of the enzyme that synthesizes hyaluronic acid?
Hyaluronic acid is synthesized by a class of integral membrane proteins called hyaluronan synthases, of which vertebrates have three types: HAS1, HAS2, and HAS3. These enzymes lengthen hyaluronan by repeatedly adding D -glucuronic acid and N -acetyl- D -glucosamine to the nascent polysaccharide as it is extruded via ABC-transporter through the cell membrane into the extracellular space. The term fasciacyte was coined to describe fibroblast-like cells that synthesize HA.
How is hyaluronic acid degraded?
Hyaluronic acid can be degraded by a family of enzymes called hyaluronidases. In humans, there are at least seven types of hyaluronidase-like enzymes, several of which are tumor suppressors. The degradation products of hyaluronan, the oligosaccharides and very low-molecular-weight hyaluronan, exhibit pro- angiogenic properties. In addition, recent studies showed hyaluronan fragments, not the native high-molecular weight molecule, can induce inflammatory responses in macrophages and dendritic cells in tissue injury and in skin transplant.
How does hyaluronic acid help with wound healing?
As of 2016, however, reviews of its effect on wound healing in burns, diabetic foot ulcers or surgical skin repairs show only limited positive clinical research evidence. Hyaluronic acid combines with water and swells to form a gel, making it useful in skin treatments as a dermal filler for facial wrinkles; its effect lasts for about 6 to 12 months, and treatment has regulatory approval from the US Food and Drug Administration.
What is the role of hyaluronic acid in synovial fluid?
For example, hyaluronic acid is a major component of the synovial fluid, and was found to increase the viscosity of the fluid. Along with lubricin, it is one of the fluid's main lubricating components. Hyaluronic acid is an important component of articular cartilage, where it is present as a coat around each cell ( chondrocyte ).
When was hyaluronic acid first used?
Hyaluronic acid was first obtained by Karl Meyer and John Palmer in 1934 from the vitreous body in a cow's eye. The first hyaluronan biomedical product, Healon, was developed in the 1970s and 1980s by Pharmacia, and approved for use in eye surgery (i.e., corneal transplantation, cataract surgery, glaucoma surgery, and surgery to repair retinal detachment ). Other biomedical companies also produce brands of hyaluronan for ophthalmic surgery.
How to calculate pH and pOH?
Calculating pH and pOH. To calculate pH from [H+] pH= -log [H+] To calculate pOH from [OH-] pOH= -log [OH-] To calculate pOH from pH. pOH=14-pH.
What does the pH scale indicate?
The pH scale, as shown in the picture above, is a way to indicate the STRENGTH of an acid. The strength of an acid refers to its tendency or ability to lose an H+ proton. This isa ability to lose a proton determined by the acid's ability to completely DISSOCIATE to form hydrogen ions.
What does it mean when a base is 7?
Bases on the pH scale. If a substance is 7 on the pH scale, it is neutral, meaning it is neither an acid nor a base (water, for example, is neutral). When a substance is higher than a 7 on a pH scale, the substance is basic. A weak base is assigned a pH between 8 and 10, while a strong base is assigned a pH between 11 and 14.
What is the pH of a strong acid?
All acids are below 7 on the pH scale. Strong acids are assigned a pH between 1 and 3, while weak acids are assigned a pH of 4 through 6.
How much hyaluronic acid is in eye drops?
For dry eye: Eye drops (Hyalistil, Hyalein, New Hyaluni, Hyaluni, Visaid) containing 0.1% to 0.3% hyaluronic acid have been used 3-8 times daily. For swelling (inflammation) and sores inside the mouth (oral mucositis): Hyaluronic acid (Gelclair, Helsinn Healthcare SA) can be mixed with water and used as a mouth rinse.
How long does hyaluronic acid help with wrinkles?
Some research shows that injecting a specific hyaluronic acid medical device (Juvéderm Ultra Plus, Allergan) into facial wrinkles can reduce wrinkles for up to one year. Hyaluronic acid has also been taken by mouth in combination with other ingredients.
What is the best treatment for sores in the mouth?
Swelling ( inflammation) and sores inside the mouth (oral mucositis). Hyaluronic acid is effective for treating mouth sores when applied as a gel or used as a rinse.
Where is hyaluronic acid found?
Hyaluronic acid is a substance that is naturally present in the human body. It is found in the highest concentrations in fluids in the eyes and joints. The hyaluronic acid that is used as medicine is extracted from rooster combs or made by bacteria in the laboratory.
What is the purpose of the CONDITIONS OF USE AND IMPORTANT INFORMATION?
CONDITIONS OF USE AND IMPORTANT INFORMATION: This information is meant to supplement, not replace advice from your doctor or healthcare provider and is not meant to cover all possible uses, precautions, interactions or adverse effects. This information may not fit your specific health circumstances.
Can hyaluronic acid be injected into joints?
Hyaluronic acid can be injected into the joint by a healthcare provider to reduce joint pain and stiffness. Hyaluronic acid is approved by the FDA as a medical device for this purpose. But not all people seem to benefit from this treatment. Also, any improvement is usually short-term.
Can hyaluronic acid be used for eye pain?
It is also approved as an injection into the eye for patients with cataracts. People use hyaluronic acid for various joint disorders, urinary tract infections ( UTIs ), acid reflux, dry eyes, vaginal pain, aging, and many other conditions, but there is no good scientific evidence to support these uses.
What is the difference between a boric acid and a boiling water reactor?
Boric acid is used only in pressurized water reactors (PWRs) whereas boiling water reactors (BWRs) employ control rod pattern and coolant flow for power control .
What is the composition of boron?
Natural boron consists of approximately 20% boron-10 and 80% boron-11 isotopes. Boron-10 has a high cross-section for absorption of low energy (thermal) neutrons. By increasing boric acid concentration in the reactor coolant, the probability that a neutron will cause fission is reduced.
What is the preservative in urine sample bottles?
The preservative in urine sample bottles in the UK is boric acid.
How does boric acid affect nuclear power plants?
Boric acid is used in some nuclear power plants as a neutron poison. The boron in boric acid reduces the probability of thermal fission by absorbing some thermal neutrons. Fission chain reactions are generally driven by the probability that free neutrons will result in fission and is determined by the material and geometric properties of the reactor. Natural boron consists of approximately 20% boron-10 and 80% boron-11 isotopes. Boron-10 has a high cross-section for absorption of low energy (thermal) neutrons. By increasing boric acid concentration in the reactor coolant, the probability that a neutron will cause fission is reduced. Changes in boric acid concentration can effectively regulate the rate of fission taking place in the reactor. Boric acid is used only in pressurized water reactors (PWRs) whereas boiling water reactors (BWRs) employ control rod pattern and coolant flow for power control. BWRs use an aqueous solution of boric acid and borax or sodium pentaborate for an emergency shut down system. Boric acid may be dissolved in spent fuel pools used to store spent fuel elements. The concentration is high enough to keep neutron multiplication at a minimum. Boric acid was dumped over Reactor 4 of the Chernobyl nuclear power plant after its meltdown to prevent another reaction from occurring.
What is borax used for?
Boric acid is added to borax for use as welding flux by blacksmiths. Boric acid, in combination with polyvinyl alcohol (PVA) or silicone oil, is used to manufacture Silly Putty. Boric acid is also present in the list of chemical additives used for hydraulic fracturing (fracking) in the Marcellus Shale in Pennsylvania.
How much boric acid is in LD 50?
The Fourteenth Edition of the Merck Index indicates that the LD 50 of boric acid is 5.14 g/kg for oral dosages given to rats, and that 5 to 20 g/kg has produced death in adult humans. For comparison's sake, the LD 50 of salt is reported to be 3.75 g/kg in rats according to the Merck Index.
What is the chemical formula for boric acid?
It has the chemical formula H 3 B O 3 (sometimes written B (OH) 3 ), and exists in the form of colorless crystals or a white powder that dissolves in water.
Overview
Hyaluronic acid , also called hyaluronan, is an anionic, nonsulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. It is unique among glycosaminoglycans as it is non-sulfated, forms in the plasma membrane instead of the Golgi apparatus, and can be very large: human synovial HA averages about 7 million Da per molecule, or about 2…
Physiological function
Until the late 1970s, hyaluronic acid was described as a "goo" molecule, a ubiquitous carbohydrate polymer that is part of the extracellular matrix. For example, hyaluronic acid is a major component of the synovial fluid and was found to increase the viscosity of the fluid. Along with lubricin, it is one of the fluid's main lubricating components.
Medical uses
Hyaluronic acid has been FDA-approved to treat osteoarthritis of the knee via intra-articular injection. A 2012 review showed that the quality of studies supporting this use was mostly poor, with a general absence of significant benefits, and that intra-articular injection of HA could possibly cause adverse effects. A 2020 meta-analysis found that intra-articular injection of high molecular weight HA improved both pain and function in people with knee osteoarthritis.
Structure
Hyaluronic acid is a polymer of disaccharides, which are composed of D-glucuronic acid and N-acetyl-D-glucosamine, linked via alternating β-(1→4) and β-(1→3) glycosidic bonds. Hyaluronic acid can be 25,000 disaccharide repeats in length. Polymers of hyaluronic acid can range in size from 5,000 to 20,000,000 Da in vivo. The average molecular weight in human synovial fluid is 3–4 million Da, and hyaluronic acid purified from human umbilical cord is 3,140,000 Da; other source…
Biological synthesis
Hyaluronic acid is synthesized by a class of integral membrane proteins called hyaluronan synthases, of which vertebrates have three types: HAS1, HAS2, and HAS3. These enzymes lengthen hyaluronan by repeatedly adding D-glucuronic acid and N-acetyl-D-glucosamine to the nascent polysaccharide as it is extruded via ABC-transporter through the cell membrane into the extracellular space. The term fasciacyte was coined to describe fibroblast-like cells that synthes…
Biosynthetic mechanism
Hyaluronic acid (HA) is a linear glycosaminoglycan (GAG), an anionic, gel-like, polymer, found in the extracellular matrix of epithelial and connective tissues of vertebrates. It is part of a family of structurally complex, linear, anionic polysaccharides. The carboxylate groups present in the molecule make it negatively charged, therefore allowing for successful binding to water, and making it valuable to cosmetic and pharmaceutical products.
Degradation
Hyaluronic acid can be degraded by a family of enzymes called hyaluronidases. In humans, there are at least seven types of hyaluronidase-like enzymes, several of which are tumor suppressors. The degradation products of hyaluronan, the oligosaccharides and very low-molecular-weight hyaluronan, exhibit pro-angiogenic properties. In addition, recent studies showed hyaluronan fragments, not the native high-molecular weight molecule, can induce inflammatory responses i…
Etymology
Hyaluronic acid is derived from hyalos (Greek for vitreous, meaning ‘glass-like’) and uronic acid because it was first isolated from the vitreous humour and possesses a high uronic acid content. The term hyaluronate refers to the conjugate base of hyaluronic acid. Since the molecule typically exists in vivo in its polyanionic form, it is most commonly referred to as hyaluronan.