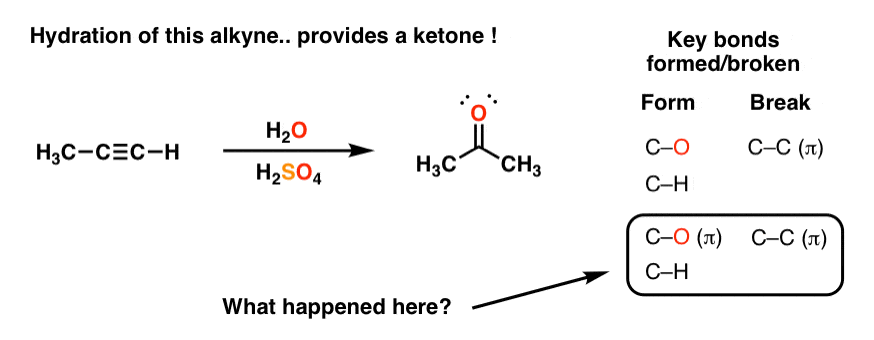
In the equation -log [H3O+], the H3O is referring to the entire molecule of the H+ being bonded to the molecule of water. Meanwhile, in -log [H+], the H is referring specifically to the Hydrogen atom that is being bonded with the H3O molecule. The concentrations of H+ and H3O+ would be equal, so these equations are essentially the same.
What is the difference between H3O+ and H+ ion?
2 Answers. The H3O+ ion is considered to be the same as the H+ ion as it is the H+ ion joined to a water molecule. The proton cannot exist in aqueous solution, due to its positive charge it is attracted to the electrons on water molecules and the symbol H3O+ is used to represent this transfer.
What is the product of H3O+ and Oh-?
The product of [H3O+] = [OH-] is the ionic product of water. shows that in aqueous (water) solutions, whether acidic, basic or neutral, the product of the ion concentrations equals 10^-14. Acidic solutions contain more H3O+ ions than OH- ions.
Why H+ ion does not exist on its own in H2O?
The H+ ion is a lone proton with a powerful charge. It does not exist on its own in an aqueous solution because it is immediately attracted to the unshared or paired electron in the oxygen atom of H2O. The result is Hydronium (H3O+). This process is reversible.
What is the H3O+ ion concentration in pure water at 25°C?
The H3O+ ion concentration in pure water at 25° C is 10^-7 dm^-3. This can be written as: [H3O+] = 10^-7 where the symbol [ ] means the "molarity of" (units in moles dm^-3).
Which solution contains more H3O+ ions than OH- ions?
What is the concentration of H3O+ in water?
Why can't a proton be in an aqueous solution?
What ions do acids and bases produce?
How many lone pairs does oxygen have?
Is pure water acidic or basic?
See 3 more
About this website
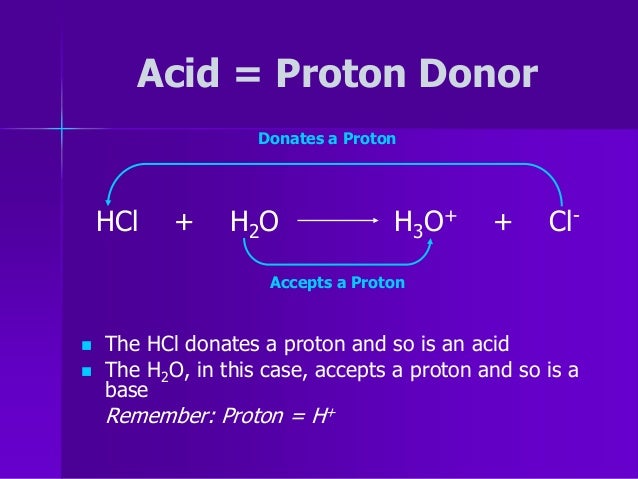
How do you find H+ from H3O+?
1:002:20Calculating the H+ or H3O+ using pH or pOH - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo we know that pH equals a negative log of the concentration. Of H+ or in this case h3o plus so toMoreSo we know that pH equals a negative log of the concentration. Of H+ or in this case h3o plus so to figure this out what we have to do is make both sides we have to multiply both sides by negative.
Is Hydronium the same as H?
Hydrogen ion is shown by the symbol H+ and hydronium ion is denoted by the symbol H3O+. Hydrogen ion is obtained by removing an electron from the hydrogen atom. Since this is so reactive, in aqueous medium it combines with water, to form a hydronium ion. Hydronium ions are also generated by protonation of water.
Is H+ and H3O+ Same?
The H3O+ ion is considered to be the same as the H+ ion as it is the H+ ion joined to a water molecule. The proton cannot exist in aqueous solution, due to its positive charge it is attracted to the electrons on water molecules and the symbol H3O+ is used to represent this transfer.
What ion is H+?
hydrogen ionhydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. The hydrogen nucleus is made up of a particle carrying a unit positive electric charge, called a proton. The isolated hydrogen ion, represented by the symbol H+, is therefore customarily used to represent a proton.
What is H called in chemistry?
hydrogenhydrogen (H), a colourless, odourless, tasteless, flammable gaseous substance that is the simplest member of the family of chemical elements.
What does H+ represent in chemistry?
H+ = Proton It becomes the positively charged hydrogen ion known as H+. This is the form of Hydrogen that produces the ATP enzyme that powers our cells and mitochondria. The H+ hydrogen ion is the basis of the pH scale. H:– = Hydride.
Why is H2 not H?
In other words, two hydrogen atoms (H) are covalently bonded (a type of chemical bond) together as H-H. Because there are two hydrogen atoms, we call this diatomic hydrogen, di meaning two. Because the hydrogen atoms are covalently bonded together they form a molecule; so H2 is also referred to as molecular hydrogen.
What is the symbol for hydronium?
H₃O⁺Hydronium / Formula
Why do some chemistry textbooks use H+ while others use H3O ... - Quora
Answer (1 of 16): That’s an interesting question. This is how I understand it. H+ does not exist in our world outside of particle accelerators, and also in space. It is really a hot entity. It will instantaneously react with the first atom it encounters to dissipate that concentrated cloud of ene...
(PDF) The solvated proton is NOT H3O+! - ResearchGate
ABSTRACT: For some time now, there has been disagreement concerning the best way to denote the aqueous proton in chemical equations. Recent results have shown that although the hydronium ion, H 3 ...
How do you calculate the PH of pure water at 50 degrees?
Can someone help me with this, the answer says 13.3 but some people say its 6.6 You sumhow use Kw to find the answer but i dont know how. Could someone sho
3.4.3 Acids and Bases - The ionic product of water, Kw - A level chem
However, as the concentration of the water effectively remains constant on both sides of the equilibrium then the [H 2 O] term can be removed to a very close approximation and the equilibrium constant denoted as Kw (sometimes called the ionic product of water).This gives: Kw = [H +][OH-]. The constant Kw remains unchanged at constant temperature (as all good constants should!).
H vs H3O
I've always seen acidity written as -log [H+]. Is this different than -log [H3O+]?
H vs H3O
I've always seen acidity written as -log [H+]. Is this different than -log [H3O+]?
Which solution contains more H3O+ ions than OH- ions?
Acidic solutions contain more H3O+ ions than OH- ions. For basic solutions it is the reverse.
What is the concentration of H3O+ in water?
The ionic component is at a very low concentration and a water molecule is generally considered covalent with a dipole moment favouring a slight positive charge. The H3O+ ion concentration in pure water at 25° C is 10^-7 dm^-3. This can be written as: [H3O+] = 10^-7.
Why can't a proton be in an aqueous solution?
The proton cannot exist in aqueous solution, due to its positive charge it is attracted to the electrons on water molecules and the symbol H3O+ is used to represent this transfer . The equation can be written as: H+ + H2O (l) → H3O+ (aq). This is hydrolysis as it is involving water as a reactant.
What ions do acids and bases produce?
In all cases, acids yield protons ( or hydronium ions H3O+) and bases yield OH- (hydroxide) ions in aqueous solutions.
How many lone pairs does oxygen have?
If you know, the oxygen atom in water contains two lone pairs. When it donates one of the lone pairs to the hydrogen atom which doesn't have any electrons, you get H X 3 O X +. In all cases, acids yield protons ( or hydronium ions H3O+) and bases yield OH- (hydroxide) ions in aqueous solutions.
Is pure water acidic or basic?
This shows that pure water is neither acidic or basic, it is neutral. The product of [H3O+] = [OH-] is the ionic product of water. shows that in aqueous (water) solutions, whether acidic, basic or neutral, the product of the ion concentrations equals 10^-14. Acidic solutions contain more H3O+ ions than OH- ions.
Why does hydrogen rarely exist on its own?
However an atom of hydrogen rarely exists on its own because its unpaired proton eagerly seeks to join up with an electron stealing life-force energy from the body.
What does pH stand for in chemistry?
pH stands for potential of Hydrogen and is actually a measurement of the concentration of hydrogen ions (H+) in a solution. Water breaks down (dissociates) into protons (H+) and hydroxides (OH–). This reaction is reversible.
What is the name of the ion that is a lone proton?
A water molecule (H20) plus a hydrogen ion (H+) becomes a hydronium ion (H3O+). The H+ ion is a lone proton with a powerful charge. It does not exist on its own in an aqueous solution because it is immediately attracted to the unshared or paired electron in the oxygen atom of H2O. The result is Hydronium (H3O+). This process is reversible. Two water molecules can disassociate to form hydronium plus hydroxide.
What does pH mean in water?
pH indicates whether water is proton saturated or acidic or an electron donor or energy donor or alkaline. More H+ = more protons or acids. Less H+ = more electrons or more alkalinity.
How does the pH scale work?
The pH scale is logarithmic. Increasing by 1 on the pH scale results in a 10 times decrease in the proton ion concentration and increasing by 3 on the pH scale results in a 1,000 times decrease in the proton ion concentration.
What is the chemical compound that binds to oxygen?
Hydride (:– ) also binds to Oxygen (O+) to form chemical compounds which are reducing agents or what is referred as an anti-acid or anti-oxidant which means against acid.
Is H+ a promiscuous ion?
Experiments indicate that the proton (H+) is very promiscuous or damaging to DNA. It changes from one H2O partner to another many times per second creating a new H3O+ ion as it moves.
Which solution contains more H3O+ ions than OH- ions?
Acidic solutions contain more H3O+ ions than OH- ions. For basic solutions it is the reverse.
What is the concentration of H3O+ in water?
The ionic component is at a very low concentration and a water molecule is generally considered covalent with a dipole moment favouring a slight positive charge. The H3O+ ion concentration in pure water at 25° C is 10^-7 dm^-3. This can be written as: [H3O+] = 10^-7.
Why can't a proton be in an aqueous solution?
The proton cannot exist in aqueous solution, due to its positive charge it is attracted to the electrons on water molecules and the symbol H3O+ is used to represent this transfer . The equation can be written as: H+ + H2O (l) → H3O+ (aq). This is hydrolysis as it is involving water as a reactant.
What ions do acids and bases produce?
In all cases, acids yield protons ( or hydronium ions H3O+) and bases yield OH- (hydroxide) ions in aqueous solutions.
How many lone pairs does oxygen have?
If you know, the oxygen atom in water contains two lone pairs. When it donates one of the lone pairs to the hydrogen atom which doesn't have any electrons, you get H X 3 O X +. In all cases, acids yield protons ( or hydronium ions H3O+) and bases yield OH- (hydroxide) ions in aqueous solutions.
Is pure water acidic or basic?
This shows that pure water is neither acidic or basic, it is neutral. The product of [H3O+] = [OH-] is the ionic product of water. shows that in aqueous (water) solutions, whether acidic, basic or neutral, the product of the ion concentrations equals 10^-14. Acidic solutions contain more H3O+ ions than OH- ions.