
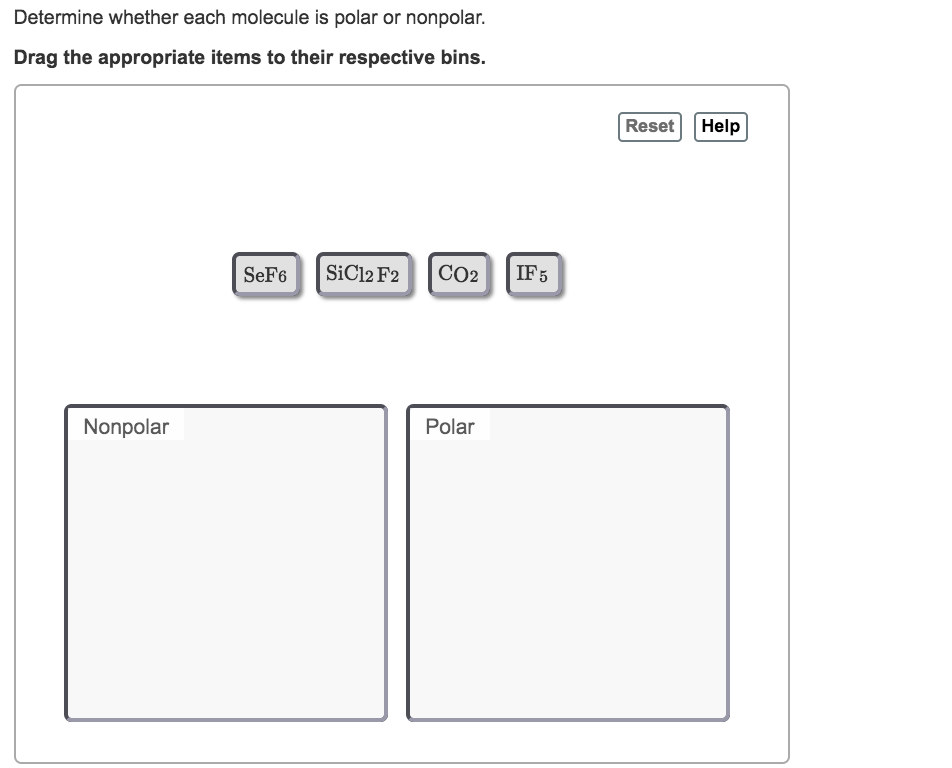
Which molecule has a polar bond, but is nonpolar in nature?
Molecules in one or more atoms have more than eight electrons (e.g., SF6). Molecules with less than eight electrons; an example is the BF3. There are molecules with a polar bond, but the molecular geometry is symmetrical. As a result, they are nonpolar molecules by nature (examples: CO2, SO3).
What are examples of non polar molecules?
Examples of Polar and Non-Polar Molecules
- In general, pyramid-shaped and V-shaped molecules are said to be polar. ...
- Water is said to be a polar molecule due to the difference in the electronegativities between the oxygen atom and the hydrogen. ...
- Fats, petrol, oil, gasoline are said to be non-polar molecules as they do not dissolve in water and nonpolar is insoluble in water.
What is the difference between polar and non - polar substances?
Summary:
- Polar substances have enough positive and negative electrical charges on the atoms while non-polar substances do not have abundant charges.
- Polar substances will mix with polar substances but polar substances will not mix with non-polar substances.
- An example of polar substances are water and alcohol. An example of non-polar is oil.
How does molecule that has polar bonds be nonpolar?
Polar molecules occur when there is an electronegativity difference between the bonded atoms. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out.
What is a polar molecule?
How can you differentiate between polar and non-polar molecules?
What do you mean by polarity?
How to determine polarity of a molecule?
What happens when sulfur pulls electrons from hydrogen?
Why is the dipole moment created between the H-S bonds?
What is the factor dipole?
See 2 more

Why is H2S not polar?
The entire molecule of hydrogen sulphide is nonpolar because it is made up entirely of non-polar $ H - S $ bonds. Despite its asymmetrical molecular shape, the molecule as a whole is non-polar due to the lack of polar links. Note : The different types of covalent bonding are mostly determined by electronegativity.
What type of bond is H2S?
covalentThe bonds of H2S are covalent because hydrogen has electronegativity about 2.2, and sulfur 2.56. Because hydrogen has smaller electronegativity it is reducer and sulfur oxidizer. However their difference is smaller, the two have electronegativities for non -metals.
Why is H2S molecule polar?
H2S is a slightly polar molecule because of the small difference in electronegativity values of Hydrogen (2.2) and Sulfur (2.58) atoms.
Why is H2S nonpolar and h2o polar?
Oxygen is more polar than Sulfur. Therefore, the Hydrogen Oxygen bond is more polar than the Hydrogen Sulfur bond. H20 has stronger intermolecular dipole--dipole interaction because of the greater polarity (or a hydrogen bond). ... H2S is bent, and bent at a smaller angle than is water.
Is H2S polar covalent or purely covalent?
The electronegativity difference, which is 0.4, suggests that H-S bonds in hydrogen sulfide are polar covalent.
Is H2S is ionic or covalent?
covalent compoundBoth H atoms remain covalently bonded to the S atom by two single bonds, as shown below: Hence, H2S H 2 S is a covalent compound.
Is H2S polar or nonpolar quizlet?
Terms in this set (10) The compound H2S contains polar bonds but the molecule is nonpolar.
How do you find the polarity of H2S?
0:041:18Is H2S Polar or Nonpolar? - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe have a negative and a positive Pole. If we have two poles that means we have a polar molecule. SoMoreWe have a negative and a positive Pole. If we have two poles that means we have a polar molecule. So h2s is a polar molecule.
What is true about H2S?
It is highly flammable and toxic, even at low concentrations. It is heavier than air and may travel along the ground.
Is H2S a more polar solvent than h2o?
H2O is more polar than H2S. Greater the electronegativity difference, greater is the polarity.
Why H2S is gas and h2o is water?
So, lesser energy is required to overcome the forces of interaction between the hydrogen sulphide molecules than those between water molecules. This energy is available at room temperature and hence, hydrogen sulphide is a gas, while water is still a liquid.
Does H2S bond with water?
Chem. Int. Ed. H2O (top) and H2S (bottom) molecules can both form hydrogen bonds.
Is H2S a hydrogen bond?
For example, consider hydrogen sulfide, H2S, a molecule that has the same shape as water but does not contain hydrogen bonds. Due to its relatively weak intermolecular forces, H2S boils at about −60 °C and so is a gas at room temperature.
Is H2S a dipole-dipole?
Since the permanent dipole moment is non zero, H2S will show dipole-dipole interactions.
What is hydrogen sulfide?
Hydrogen sulfide is most commonly encountered as a product of the anaerobic respiration of sulfidogenic organisms. For instance, some bacteria that operate in the absence of oxygen use sulfate ions (SO 4–) as the terminal electron acceptor during cellular respiration which reduces it into H 2 S. In other words, sulfidogenic organisms breathe sulfur ...
How many atoms are in hydrogen sulfide?
Hydrogen sulfide is a triatomic (3-atom) molecule that consists of a central sulfur atom and 2 terminal hydrogen atoms. Like a molecule of water, hydrogen sulfide has a bent geometric structure with a bond angle of 92.1° and bond lengths of 136 picometers (1 picometer = 1 trillionth of a meter). It is a bit denser than air and is explosive in ...
How does hydrogen sulfide affect the body?
The symptoms of hydrogen sulfide poisoning are similar to those of carbon monoxide poisoning; fatigue, dizziness, inability to concentrate, loss of memory, and irritability. Though initially a pungent odor, the body quickly acclimates to the smell, which can make people unaware of its presence. It is slightly denser than air, so it has a tendency to accumulate near the bottom of poorly ventilated spaces. The human body can tolerate low concentrations of hydrogen sulfide for some time. In high concentrations, inhalation of hydrogen sulfide can be immediately fatal or cause serious brain damage.
How does polarity work?
When two atoms form a covalent bond, they do so by sharing valence electrons. Each element has an electronegativity which is a measure of how hard they pull on electrons. When two elements that differ greatly in their electronegativities form a covalent bond, the more electronegative element will pull harder of the shared electrons than the less electronegative element. The result is that the shared electrons are pulled closer to the more electronegative element.
What is the reaction of sulfur dioxide?
It reacts with metal ions to form metal sulfides, most commonly with lead (Pb) to form lead (II) sulfide (PbS).
How is hydrogen sulfide produced?
Under the presence of heat and pressure, metal sulfide compounds will undergo hydrolysis with water to form a metal oxide and hydrogen sulfide gas. As such, hydrogen sulfide is a natural product of the process that creates natural gas. In fact, a large amount of hydrogen sulfide is produced via the separation of it from natural gas deposits. Similar mechanisms also result in the formation of hydrogen sulfide in thermal ocean vents.
When two elements that differ greatly in their electronegativities form a covalent bond, what happens?
When two elements that differ greatly in their electronegativities form a covalent bond, the more electronegative element will pull harder of the shared electrons than the less electronegative element. The result is that the shared electrons are pulled closer to the more electronegative element.
Why is H2S polar?
H2S is a slightly polar molecule because of the small difference in electronegativity values of Hydrogen (2.2) and Sulfur (2.58) atoms. In addition, the presence of two lone pairs that are on the opposite side of the two Hydrogen atoms also makes the molecule more polar and causes bent shape geometrical structure of H 2 S.
Why is hydrogen sulfide polar?
Hydrogen sulfide is polar because of the presence of lone pair of electrons in Sulfur and the electronegativity difference between Sulfur and H atoms. There are eight valence electrons present in the molecule of hydrogen sulfide. Hydrogen sulfide molecule has an angular geometry with a non-zero dipole moment.
What is the polarity of NH3?
The polarity of the NH3 molecule is due to the electronegativity difference between N (3.04) and H ( (2.2).
How to determine if a molecule is polar or nonpolar?
Polarity is determined by electronegativity. A molecule is polar if the structure of that molecule is not symmetric. In the case of symmetric structure, the dipole vectors on each molecule cancel each other, resulting in the nonpolar nature of the molecule. H 2 O is another example of a polar molecule.
What is the hybridization of hydrogen sulfide?
The hybridization of the Hydrogen sulfide molecule is sp3. The Sulfur atom is in center bonding with two H atoms forming the bond angle less than 180 degrees. According to the VSEPR theory, the lone pairs of electrons repel each other. There are two lone pairs of the sulfur atom in the Hydrogen sulfide lewis structure.
How many valence electrons does the H atom have?
Hence there are two valence electrons for the H atom ( as there are two H atoms).
How many hydrogen atoms are in the H2S Lewis structure?
In the H2S Lewis structure, there are two hydrogen atoms on both sides of the central sulfur atom.
Why is H2S a polar molecule?
Due to the existence of two lone pairs on the Sulfur atom, the hydrogen sulfide (H2S) molecule has a twisted V- shape bent form. According to the VSEPR hypothesis, lone pairs and bond pairs repel each other, causing the S-H bonds to move the lower side of the molecular structure, resulting in a V-shaped molecule.
Why is H2O polar but H2S is not?
Because the oxygen atom in water is very electronegative and can partially polarise the hydrogen atoms, hydrogen-hydrogen bonds can form between the H2O molecules, resulting in a relatively high boiling point. Because sulphur is substantially less electronegative than oxygen, these bonds do not occur in H2S.
What are H2S electron and molecular geometry?
H2S has a V-shaped bent molecular geometry and water like electron geometry, according to the VSEPR theory. Because the core central atom, sulfur, has two S-H bonds with the surrounding two hydrogen atoms. In the same plane, the H-S-H bond forms a 92-degree angle. Because two hydrogen atoms are in the same plane, they form a V- type bent shape.
what are the electron and molecular geometries, respectively, for hydrogen sulfide, H2S?
H2S molecule, electron geometry is tetrahedral with sp3 hybridization and molecular geometry is V- shape bent structure.
How many electrons does sulfur have?
Sulfur is the middle element of the molecule, with 6 electrons in its outermost valence electron shell, while hydrogen atom is the outermost valence electron shell, with one electron.
What is the bond between sulfur and hydrogen?
As a result of this, both two hydrogen atoms form covalent bonds with the sulfur atom, leaving the sulfur atom with two lone pairs. The bond pairs of S-H are repelled by the two lone pairs on the sulfur atom. According to VSEPR theory, electronic repulsion causes the molecule’s shape to bend (V-shape), similar to that of the water molecule.
Why is the dipole moment of H2S important?
The polarity of any molecule is proportional to its dipole moment. Because the form of H2S is asymmetric. The dipole moment of H2S does not cancel each other as a result of this.
What is a polar molecule?
Since a molecule is neutral but it is called as polarised when one side is more negative charge than the other positive-charged side.
How can you differentiate between polar and non-polar molecules?
The polar molecules have an unequal sharing of electrons i.e. the charges are not balanced. But in non-polar molecules , there are relatively equal numbers of electrons.
What do you mean by polarity?
Polarity is described as how electrons are distributed in the molecule. It shows wherewith electrons are attracted and pulled by the most electronegative atom.
How to determine polarity of a molecule?
To determine the polarity of any molecule like H2S, it is equally important to find out its outside atoms, and shape. There are two lone pairs of electrons on the central atom Sulfur that causes the H-S bond to be in a bent shape. Hence, the molecule has an odd distribution of atoms around the central atom making it non-symmetrical. ...
What happens when sulfur pulls electrons from hydrogen?
With atomic number 16, Sulfur pulls both the electrons of Hydrogen to complete its last shell and gains a negative charge. Hydrogen becomes positive-charged.
Why is the dipole moment created between the H-S bonds?
Hence, the molecule has an odd distribution of atoms around the central atom making it non-symmetrical. Because of its bent shape , the dipole moment is created between the H-S bonds. The greater the separation of charges more is the dipole moment between the atoms.
What is the factor dipole?
The factor dipole of a molecule shows the level of its polarity. Greater the polarity of a molecule more is the value of its dipole moment.
Polarity in A Nutshell
The Polarity of Hydrogen Sulfide
- Applying the previous lesson on polarity, we can find out if hydrogen sulfide is a polar compound. Hydrogen has an EN value of 2.1 and sulfur has an EN value of 2.5. the difference between these two values is less than 0.5, so H-S bonds are classified as non-polar. Since hydrogen sulfide consists entirely of non-polar H-S bonds, the entire molecule is non-polar. Strictly speaking, H-S …
Hydrogen Sulfide as A Compound
- Hydrogen sulfide is a triatomic (3-atom) molecule that consists of a central sulfur atom and 2 terminal hydrogen atoms. Like a molecule of water, hydrogen sulfide has a bent geometric structure with a bond angle of 92.1° and bond lengths of 136 picometers (1 picometer = 1 trillionth of a meter). It is a bit denser than air and is explosive in the presence of oxygen and heat. Hydro…
Occurrences of Hydrogen Sulfide
- Anaerobic Respiration One of the primary natural sources of hydrogen sulfide is the activity of sulfidogenic bacteria. Sulfidogenic bacteria use sulfur instead of oxygen for their metabolisms. During sulfidogenic respiration, bacteria will use sulfate ions as a reducing agent to carry electrons on the electron transport train. At the end of this reaction, the sulfate ions are reduced …
Toxicity of Hydrogen Sulfide
- In general, hydrogen sulfide is very toxicto obligate oxygen breathers. Its mechanisms of action are similar to that of carbon monoxide. Hydrogen sulfide will bind to important enzymes and cofactors, preventing them from doing their job during cellular respiration. Since hydrogen sulfide is naturally produced in the human body, the body does have mechanisms for removing hydroge…