HClO2 is a stronger acid than HClO.... HClO4, perchloric acid, is a very strong acid as is HClO3. HClO2 is a weak acid and HClO is even weaker.
Is HClO is the strongest acid?
Thus, {ClO}- will be strongest base and so its conjugate acid HClO will be the weakest acid. Similarly, in this series {ClO4}- is the weakest base (maximum stabilized) and its conjugate acid HClO4 is the strongest acid. 110V 5L Home Oxygen Concentrator is on sale!
Is HClO2 a weak electrolyte?
– All other acids are weak acids/weak electrolytes: HF, HC2H3O2, HNO2, H2SO3, etc. Is BaCl2 an electrolyte? BaCl2 is a strong electrolyte, even stronger then NaCl (difference in electronegativity of 2.23). The substances which are completely ionized in dissolved or molten state, are known as strong electrolytes. Is na2so4 an electrolyte?
Which acid is stronger between HCl or H2SO4?
This is not a completely straightforward question. HCl and H2SO4 are categorized as strong acids. That means that they are about 100% ionized in water. In water all of the strong acids are of essentially the same strength. This happens because water is a leveling solvent. In water the strongest acid that can exist is H3O+, or hydronium ion.
Why is HCl a stronger acid than acetic acid?
HCL is stronger than acetic acid because it undergoes almost complete ionisation when dissolved in water(i.e. formation of H^+ and Cl^- ion) whereas when acetic acid is dissolved in water only 5% of it is dissociated into H^+ and CH3COOH^- ions.
Which is the strongest acid * 1 point HClO4 HClO3 HClO2 HClO?
The HClO4 has the most oxygen atoms so it would have to be the strongest acid.
Which of the following is the weakest acid HCL HClO HClO2 HClO3 HClO4?
The weakest acid is HClO. Therefore the strongest conjugate base is ClO– . The acidic strength of oxoacids of chlorine is in the order 'HClO4,HClO3,HClO2,HClO' Briefly explain the reason for this.
Is HClO4 or HClO2 a stronger acid?
HClO4 is stronger acid due to the formation of more stable conjugate base. Also there are more number electronegative oxygen atoms in case of HClO4 , so acidity should be more.
Is HClO2 is a strong acid?
B. Weak Acid: dissolves but less than 100% dissociates to produce protons (H+) 1. any acid that is not one of the seven strong is a weak acid (e.g. H3PO4, HNO2, H2SO3, HClO, HClO2, HF, H2S, HC2H3O2 etc.) 2.
Which is a stronger acid HClO4 or HClO3?
HClO4 is stronger than HClO3. The oxidation number of Cl in HClO4 is +7 and in HClO3 is +5. The acid strength of oxyacid of the same halogen increases with the increase in oxidation number of halogen.
Why is HClO a strong acid?
Why is HClO₂ a stronger acid than H2SO4? Hclo2 is stronger acid than H2so4 . It is due to the stable conjugate base of Hclo2 than H2so4. Or Hclo2 is resonance stabilised and can donate H+ion more easily than H2so4.
Is HClO a strong acid?
Since HClO is not one of these seven, and there is no -OH group present as there is in bases, HClO is a weak acid.
Why is HClO2 weaker than HClO4?
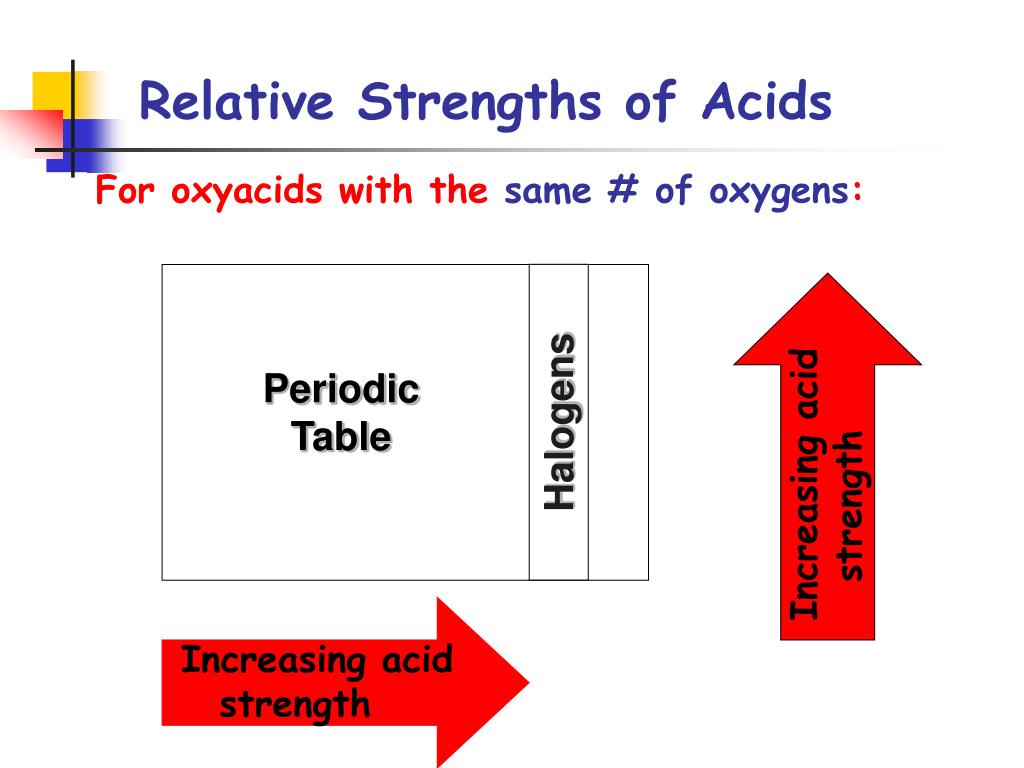
Re: Relative acidity of HClO vs HClO2 etc. As the number of oxygens increases as you go from HClO to HClO4, the oxidation number of Cl increases. The oxidation number for Cl in HClO is +1, +3 for HClO2, +5 for HClO3, and +7 for HClO4. The oxidation number represents the number of electrons Cl loses.
Why is HClO more acidic than HClO4?
Solution. HClO4 is a stronger acid than HClO. This is because ClO−4ClO4- is a weaker conjugate base than ClO−. In ClO−4ClO4-, the negative charge is spread over four oxygen atoms owing to resonance.
Why is HClO3 more acidic than HClO?
The acid is much stronger if the number of oxygen atoms is more. In HClO3 and HClO2, HClO3 has more oxygen therefore it is more acidic.
Why is HClO3 stronger than HClO?
0:117:30Explain why chloric acid HClO3 is a stronger acid than ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipIon or c because the chlorate ion clo3 is a stronger base than the chloride ion.MoreIon or c because the chlorate ion clo3 is a stronger base than the chloride ion.
Is HClO a strong or weak electrolyte?
Classifying ElectrolytesStrong Electrolytesstrong acidsHCl, HBr, HI, HNO3, HClO3, HClO4, and H2SO4strong basesNaOH, KOH, LiOH, Ba(OH)2, and Ca(OH)2saltsNaCl, KBr, MgCl2, and many, many moreWeak Electrolytesweak acidsHF, HC2H3O2 (acetic acid), H2CO3 (carbonic acid), H3PO4 (phosphoric acid), and many more1 more row
Is HClO a weak acid?
HClO is a weak acid.
Is HClO stronger than HCl?
Order in the increasing order of acidity:HCl, H2SO4, HF, HCl, HI, HBr, HNO3, HBrO, HClO, HClO3, HClO4, H2S, H3PO4(?)
Why is HClO a weak acid?
HClO is an acid as is has the proton that it can donate but it is a weak acid because it is not one the acid amoung the list of the strong acids.
Is HClO3 strong or weak?
The 7 common strong acids are: HCl, HBr, HI, HNO3, HClO3, HClO4 and H2SO4 (1st proton only).
Relative acidity of HClO vs HClO2 etc
Can someone provide more of the "why" in this explanation pasted below for the relative acidity of HClo, HClo2, etc.? My comments/questions are in italics
Re: Relative acidity of HClO vs HClO2 etc
In general, adding more oxygen atoms to the central atom in an oxyacid helps to distribute the negative charge of the conjugate base over a greater number of atoms. If a proton is less strongly attached to any one of the oxygens, then you get a stronger acid.
