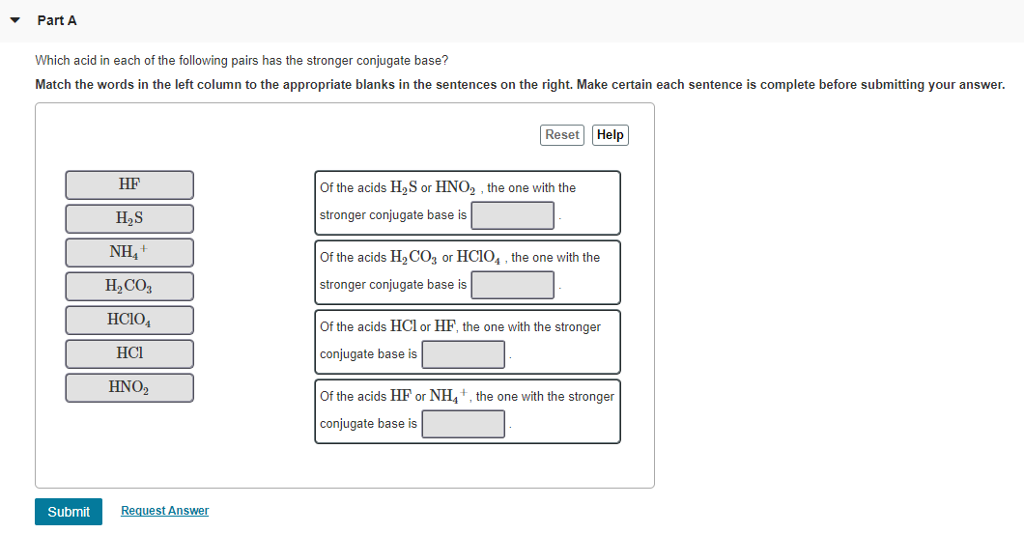
Is HNO2 a strong or weak acid?
HNO 2 is a weaker acid than HNO 3. HNO 2 (Nitrous acid) is a weak acid. Because it does ...
Is HNO2 a conjugate acid or a conjugate base?
Nitrous acid is a monoprotic acid with the chemical formula HNO 2. It is made up of three elements hydrogen, oxygen, and nitrogen. It only exists in solution and is very unstable in nature. It is used to make diazonium salts from amines. In this article, we will discuss Is HNO 2 an acid or base, Is it strong or weak? its conjugate base, etc.
Is HNO3 a stronger acid than HNO2?
Yes, HNO 3 is a stronger acid than HNO 2, one reason is that HNO 2 does not dissociate completely in an aqueous solution to liberate proton or H + ions whereas HNO 3 is nearly 100% ionized in an aqueous solution and completely dissociate into H + and NO 3– ions. Or check the conjugate base of acids to know the stability of the compound.
Is H2SO4 an acid or a base?
H2SO4 is acid in nature as in an aqueous solution it dissociates into 2H+ and SO42-, So, it has 2H+ ions that are replaceable that correspondingly shows its acidic nature, although, H2SO4 is one of the strongest acids, but in the presence of superacids, it can act as a base also.
Why HNO2 act as acid?
What is the Conjugate base of HNO2?
What are some examples of weak acids?
Is HNO2 an acid?
When does a substance behave as an acid?
Is HNO 2 stronger than HNO 3?
Is A acid stronger than B acid?
See 2 more

Can HNO2 be a base?
As per Bronsted-Lowry theory, HNO2 is acid and donates one proton to a water molecule and forms a base(NO2–) known as the conjugate base of an acid(HNO2).
How is HNO2 a weak acid?
0:282:15HNO2 + H2O (Nitrous Acid + Water) - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe see nitric hno3 but not hno2. So memorize your strong acids you'll know that this is a weak acidMoreWe see nitric hno3 but not hno2. So memorize your strong acids you'll know that this is a weak acid nitrous acid. Because it's weak it's going to only partially break apart or dissociate. Into its
What is the acidity of HNO2?
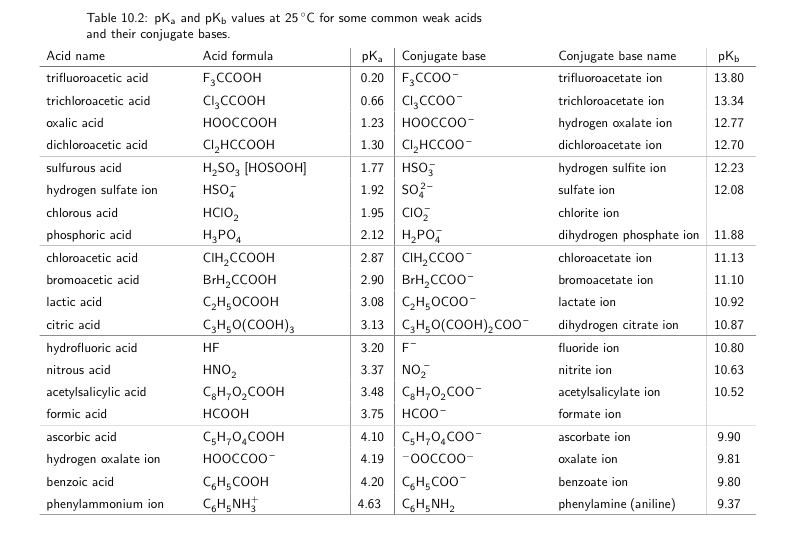
3.15Nitrous acidNamesMelting pointOnly known in solution or as gasAcidity (pKa)3.15Conjugate baseNitriteHazards32 more rows
What is HNO2 and hno3?
2. Nitric acid and nitrous acid are mono basic oxoacids of nitrogen. But they are very different in chemical and physical properties. Nitric acid is widely used in chemical industry for producing other chemicals. Also nitric acid is used as an strong acid and oxidizing acid in laboratories.
Is NO2 a weak acid?
NO2- is a weak base.
Which is a strong acid HNO2 or HNO3?
HNO3 is stronger acid than HNO2 . >>HNO3 is stronger acid than HNO2 .
Is HNO2 a strong or weak base?
any acid that is not one of the seven strong is a weak acid (e.g. H3PO4, HNO2, H2SO3, HClO, HClO2, HF, H2S, HC2H3O2 etc.)
Is HNO3 a base or acid?
HNO3 is a potent acid, a base, a nitrating agent and a heavy oxidising agent at times. In the presence of a stronger acid, it serves as a base. As the conjugate base is more stable, nitric acid is a stronger acid than nitrous acid.
Is HNO3 a weak acid?
But is HNO3 a strong acid? Yes, HNO3 is a strong acid with a pH of 3.1. The strength of an acid is determined by its dissociative property in water to produce hydronium ions (H+ or H3O+). In an aqueous solution, HNO3 acid dissociates completely into H+ and NO3- ions, therefore, considered as a strong acid.
What is the name of HNO2?
Nitrous acidNitrous acid / IUPAC ID
Is nitric acid a strong acid?
Is nitric acid a strong or weak acid? Nitric acid is a strong acid, completely ionized into hydronium (H3O+) and nitrate (NO3-) ions in an aqueous solution, and a powerful oxidizing agent.
How is HNO2 formed?
HNO2 formation happens in the presence of NO2 gas, a combustion product in the atmosphere, whereas formation from NO gas is negligible. There is no known, direct impact of HNO2 on any semiconductor process or equipment.
Is HNO2 a weak base?
any acid that is not one of the seven strong is a weak acid (e.g. H3PO4, HNO2, H2SO3, HClO, HClO2, HF, H2S, HC2H3O2 etc.)
Is HNO2 a strong or weak electrolyte?
Nitrous acid, HNO2(aq), is a weak electrolyte.
Why does HNO3 dissociate more than HNO2?
HNO3 is a stronger acid than HNO2, because: In HNO3 there is three nitrogen to oxygen bonds whereas in HNO2 there is only one. The conjugate base of HNO3 is more stable than the conjugate base of HNO2.
What is a bronsted acid?
A Bronsted acid is one that donates protons and a Bronsted base is one that accepts protons.
Is HNO2 a base or an acid?
In the example you give, HNO2 + HPO4 ^2- <=> NO2^- + H2PO4^-, HNO2 is the acid, because it loses (donates) a proton to HPO4^2-. HPO4 ^2- is the base, because it accepts the H+ from HNO2. You can distinguish bronsted acids and bases by finding their roles in proton transfer.
Why HNO2 act as acid?
An acid is something we know that donates the H + ion or proton when it is dissolved in a solution.
What is the Conjugate base of HNO2?
Conjugate acid is an acid that formed when the parent base compound gains one proton and the conjugate base is a base that formed when the parent acid compound loses one proton.
What are some examples of weak acids?
Examples of weak acids:- H 3 PO 4, CH 3 COOH, NH 4+, HF, H 2 CO 3, etc.
Is HNO2 an acid?
So, we can proudly say HNO2 is an Arrhenius acid .!
When does a substance behave as an acid?
This theory said that a substance behaves as an acid when it is ready to give off the hydrogen ion on dissolving in an aqueous solution. And a substance behaves as a base when it releases OH – ion on dissolving in an aqueous solution.
Is HNO 2 stronger than HNO 3?
HNO 2 is a weaker acid than HNO 3.
Is A acid stronger than B acid?
Note: If the conjugate base of A acid is stable than the conjugate base of B acid, then A is a stronger acid than B acid.