
Is hydrochloric acid a homogeneous mixture or compound?
So,hydrochloric acid is a homogeneous mixture but not a compound. Compound. All compounds are substances, but not all substances are compounds. What does this statement mean in simple words?
Is HCl a mixture or a solution?
A few HCl molecules remain whole and along with lots of water molecules we have a “mixture” although it should be more correctly referred to as a solution since the particles are far smaller than what is Hydrochloric acid usually comes as a solution of HCl in water, about 35% HCl, 65% water.
How is hydrochloric acid (HCl) produced?
The large-scale production of hydrochloric acid is almost always integrated with the industrial scale production of other chemicals, such as in the chloralkali process which produces hydroxide, hydrogen, and chlorine, the latter of which can be combined to produce HCl. Hydrochloric acid is produced in solutions up to 38% HCl (concentrated grade).
What is the concentration of hydrochloric acid?
Hydrochloric acid usually comes as a solution of HCl in water, about 35% HCl, 65% water. This is the most concentrated possible at normal temperatures and pressures. Pure HCl is a gas at room temperature and is a molecular substance with no H+ ions so is not considered an acid. Hydrochloric acid is made by dissolving the gas in water.
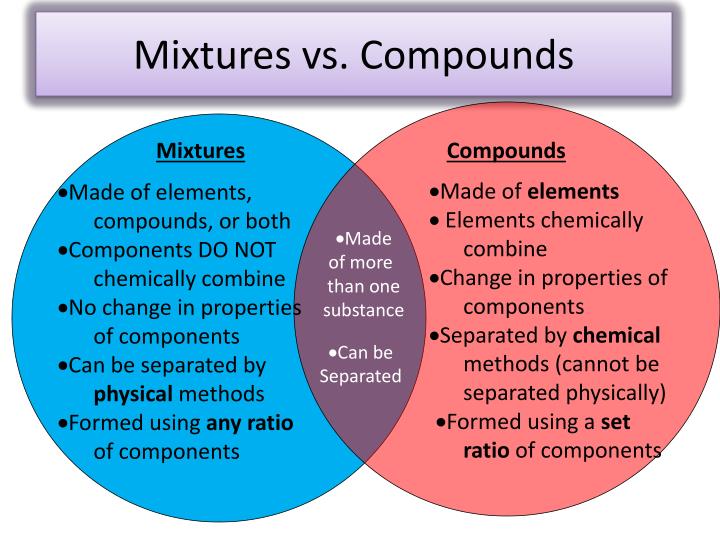
Is hydrochloric acid mixture or compound?
hydrogen chloride (HCl), a compound of the elements hydrogen and chlorine, a gas at room temperature and pressure. A solution of the gas in water is called hydrochloric acid.
Is Hydrochloric a mixture?
The chemical abbreviation of hydrochloric acid is HCl. The hydrochloric acid is a gas at room temperature and pressure. The solution of HCl gas is generally named hydrochloric acid. Therefore, hydrochloric acid is a mixture.
Is acid a compound or mixture?
Acids are chemical compounds that show, in water solution, a sharp taste, a corrosive action on metals, and the ability to turn certain blue vegetable dyes red. Bases are chemical compounds that, in solution, are soapy to the touch and turn red vegetable dyes blue.
Is hydrochloric acid a heterogeneous mixture?
Hydrochloric acid is a Homogeneous Mixture with the same amount of Chlorine and Hydrogen in fixed proportion throughout the solution.
What is a homogeneous mixture?
A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample.
What is heterogeneous mixture?
A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. By definition, a single-phase consists of a pure substance or a homogeneous mixture. There are two or more phases of a heterogeneous mixture.
What is type of mixture?
There are two types of mixtures: heterogeneous and homogeneous.
What is an example of a mixture of compounds?
Difference between Mixtures and CompoundsCompoundsMixturesExamples: Sodium Chloride, Water, Sodium Chloride, Alcohol, Petrol, etc.Example: Salt and water, Sugar and Water, Air, etc.Compounds can be considered pure substances.Mixtures are considered impure substances.8 more rows•Sep 28, 2022
What is true of a mixture?
A mixture is a combination of two or more substances in any proportions. The substances in a mixture do not combine chemically, so they retain their physical properties. A homogeneous mixture has the same composition throughout. A heterogeneous mixture varies in its composition.
Which mixture is HCl?
Azeotropic mixture of HCl and H2O has. Solution : Azeotropic mixtures are those mixtures which boils at a constant temperature and distill over completely at the same temperature without change in composition. In `HCl + H_2O` azeotropic mixture, HCl composition is 20.2% and water is 79.8%.
Which of the following are homogeneous mixture?
Soil is a homogeneous mixture.
Which of the following is not a heterogeneous mixture?
The correct answer is Air.
What is hydrochloric acid used for?
Hydrochloric acid (HCl) is commonly used for the neutralization of alkaline agents, as a bleaching agent, in food, textile, metal, and rubber industries. It is neutralized if released into the soil and it rapidly hydrolyzes when exposed to water.
What happens if you mix acid and chlorine?
When chlorine bleach is mixed with an acid, chlorine gas is given off. Chlorine gas and water combine to make hydrochloric and hypochlorous acids.
What happens if you mix hydrochloric acid with bleach?
Bleach (sodium hypochlorite) reacts with hydrochloric acid to form salt, water, and chlorine gas. The upward-pointing arrow indicates that a gas has been formed.
What happens if you mix pool acid and chlorine?
Mixing bleach, or sodium hypochlorite with muriatic acid will liberate chlorine gas, which is definitely toxic, and a severe irritant to eyes and nasal passages and the lungs even in small amounts.
How is hydrochloric acid made?
Hydrochloric acid is usually prepared industrially by dissolving hydrogen chloride in water. Hydrogen chloride can be generated in many ways, and thus several precursors to hydrochloric acid exist. The large-scale production of hydrochloric acid is almost always integrated with the industrial scale production of other chemicals, such as in the chloralkali process which produces hydroxide, hydrogen, and chlorine, the latter of which can be combined to produce HCl.
Why is hydrochloric acid called hydrochloric acid?
Because it was produced from rock salt according to the methods of Johann Rudolph Glauber, hydrochloric acid was historically called by European alchemists spirits of salt or acidum salis (salt acid). Both names are still used, especially in other languages, such as German: Salzsäure, Dutch: Zoutzuur, Swedish: Saltsyra, Spanish: Salfumán, Turkish: Tuz Ruhu, Polish: kwas solny and Czech: kyselina solná
What is the process of producing hydrochloric acid?
The large-scale production of hydrochloric acid is almost always integrated with the industrial scale production of other chemicals, such as in the chloralkali process which produces hydroxide, hydrogen, and chlorine, the latter of which can be combined to produce HCl.
What are the physical properties of hydrochloric acid?
Physical properties of hydrochloric acid, such as boiling and melting points, density, and pH, depend on the concentration or molarity of HCl in the aqueous solution. They range from those of water at very low concentrations approaching 0% HCl to values for fuming hydrochloric acid at over 40% HCl.
How much HCl is in industrial grade?
Industrial market. Hydrochloric acid is produced in solutions up to 38% HCl (concentrated grade). Higher concentrations up to just over 40% are chemically possible, but the evaporation rate is then so high that storage and handling require extra precautions, such as pressurization and cooling.
What is the process of making soda ash?
A new industrial process developed by Nicolas Leblanc of Issoudun, France enabled cheap large-scale production of sodium carbonate (soda ash). In this Leblanc process, common salt is converted to soda ash, using sulfuric acid, limestone, and coal, releasing hydrogen chloride as a by-product.
What is the name of the mineral that is a mixture of nitric acid and hydrochloric acid?
One important invention that resulted from the discovery of the mineral acids is aqua regia , a mixture of nitric acid and hydrochloric acid in a 1:3 proportion, capable of dissolving gold.

Overview
Physical properties
Physical properties of hydrochloric acid, such as boiling and melting points, density, and pH, depend on the concentration or molarity of HCl in the aqueous solution. They range from those of water at very low concentrations approaching 0% HCl to values for fuming hydrochloric acid at over 40% HCl.
Hydrochloric acid as the binary (two-component) mixture of HCl and H2O has …
History
In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi (c. 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method for producing hydrochloric acid, as perhaps manifested in the following recipe from his Kitāb al-Asrār ("The Book of Secrets"):
Chemical properties
Gaseous hydrogen chloride is a molecular compound with a covalent bond between the hydrogen and chlorine atoms. In aqueous solutions dissociation is complete, with the formation of chloride ions and hydrated hydrogen ions (hydronium ions). A combined IR, Raman, X-ray, and neutron diffraction study of concentrated hydrochloric acid showed that the hydronium ion forms hydrogen bonded complexes with other water molecules. (See Hydronium for further discussion of this issu…
Production
Hydrochloric acid is usually prepared industrially by dissolving hydrogen chloride in water. Hydrogen chloride can be generated in many ways, and thus several precursors to hydrochloric acid exist. The large-scale production of hydrochloric acid is almost always integrated with the industrial scale production of other chemicals, such as in the chloralkali process which produces hydroxide, hydrogen, and chlorine, the latter of which can be combined to produce HCl.
Applications
Hydrochloric acid is a strong inorganic acid that is used in many industrial processes such as refining metal. The application often determines the required product quality. Hydrogen chloride, not hydrochloric acid, is used more widely in industrial organic chemistry, e.g. for vinyl chloride and dichloroethane.
One of the most important applications of hydrochloric acid is in the pickling of steel, to remove r…
Presence in living organisms
Gastric acid is one of the main secretions of the stomach. It consists mainly of hydrochloric acid and acidifies the stomach content to a pH of 1 to 2. Chloride (Cl ) and hydrogen (H ) ions are secreted separately in the stomach fundus region at the top of the stomach by parietal cells of the gastric mucosa into a secretory network called canaliculi before it enters the stomach lumen.
Legal status
Hydrochloric acid has been listed as a Table II precursor under the 1988 United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances because of its use in the production of heroin, cocaine, and methamphetamine.