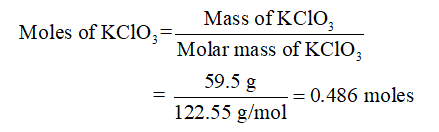
Is K2CrO4 an acid salt or base?
Is K2CrO4 an acid or base? Potassium chromate is the chromate anion’s yellow base of potassium chloride. It is a growing chemical in the laboratory, while industrial sodium chromate is essential. Why is K2CrO4 used as an indicator? An indicator that could be used is potassium chromate(VI).
Is K2CO3 an acid or a base?
Potassium carbonate (K2CO3) is a commonly used base in organic chemistry. The pKa of its conjugate acid is 10.25. It is commonly used to deprotonate moderately acidic protons such as phenols (pKa ~10) and 1,3-dicarbonyl compounds (pKa ~9-13).
Is KCl a salt base or acid?
KCl is a neutral salt. It is formed from the neutralization reaction carried out between strong acid, namely Hydrochloric acid (HCl), and strong base, namely Potassium hydroxide (KOH). Potassium chloride (KCl) is neither held acidic properties nor alkaline. Because of the absence of the two most important ions needed for acid and base nature ...
Is an alkaline solution an acid or base?
An alkaline solution is a mixture of base solids dissolved in water. The potential of hydrogen, also known as the pH scale, measures the alkalinity or acidity level of a solution. The scale ranges from zero to 14. The midpoint 7 represents a neutral pH.

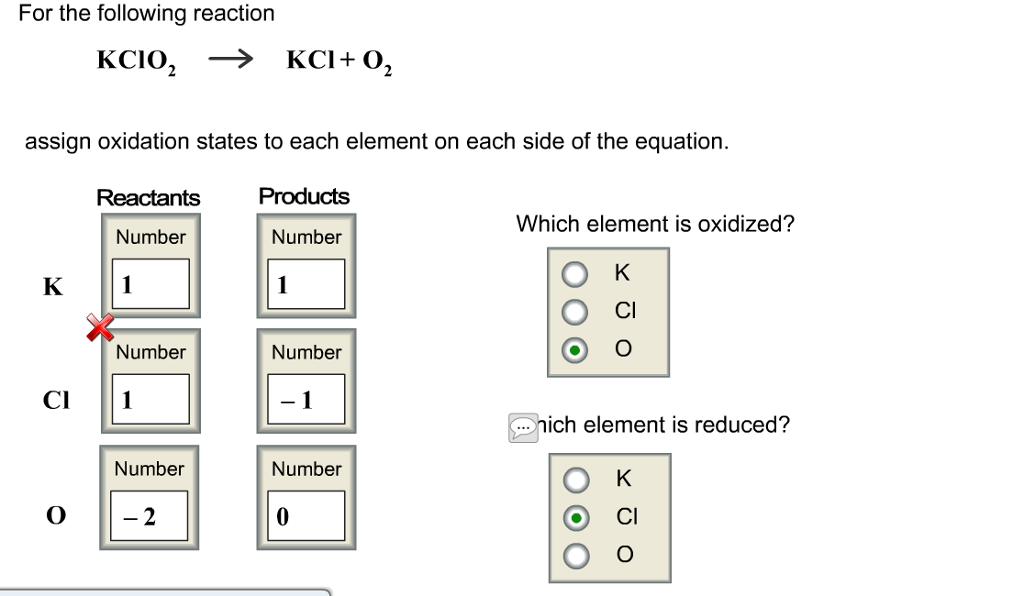
Is kclo3 base or acid?
0:046:19kclo3 acid or base - YouTubeYouTubeStart of suggested clipEnd of suggested clipKclo3 his acid or base so when we're talking about a sir bases like we said before acids againMoreKclo3 his acid or base so when we're talking about a sir bases like we said before acids again donate an electron right they donate an electron.
Is KNO3 acid or base?
KNO3 is neither acidic nor basic. It is a neutral salt because of the neutralization of a strong acid nitric acid (HNO3) with a strong base potassium hydroxide (KOH). This neutralizes each other's effects and forms a neutral solution whose pH is 7.
Is KCl neutral basic or acidic?
neutralThe KCl ions come from a strong acid (HCl) and a strong base acid (HCl) (KOH). Thus, the acidity of the solution will not be influenced by either ion, so KCl is a neutral salt.
Is KClO4 a strong acid or base?
(b) Potassium perchlorate, KClO4, is a neutral salt. Neither K+ nor ClO4- has any tendency to donate or accept a proton in dilute aqueous solutions. The reaction between a strong base KOH and the strong acid HClO4 produces KClO4.
Is kno3 a strong or weak base?
KNO3 is neither an acid nor base, it is a neutral salt as it is made from the neutralization reaction of the strong acid (HNO3) with a strong base (KOH)....Is Potassium nitrate (KNO3) an acid or base or salt?Name of MoleculePotassium nitrateNatureNeither acid nor basepH72 more rows
What is the pH of kno3?
to 7Potassium nitrate is neutral as it is a salt obtained from neutralization of a strong base and a strong acid, therefore its pH value is equal to 7.
Is nacl base or acid?
neutral saltSodium chloride, which is obtained by neutralization of hydrochloric acid and sodium hydroxide, is a neutral salt.
Is HCl base or acid?
strong acidHCl is a strong acid because it dissociates almost completely. By contrast, a weak acid like acetic acid (CH3COOH) does not dissociate well in water – many H+ ions remain bound-up within the molecule. In summary: the stronger the acid the more free H+ ions are released into solution.
Does KCl affect pH?
In this study, we demonstrate theoretically, and confirm experimentally, that KCl additions always change the pH of water samples of low solute content when compared with unspiked water samples.
Which are strong acids and bases?
Evaluate solution pH and pOH of strong acids or bases....Strong Acids.Strong AcidsStrong Baseshydrobromic acid (HBr)potassium hydroxide (KOH)hydroiodic acid (Hl)calcium hydroxide (Ca(OH)2)nitric acid (HNO3)strontium hydroxide (Sr(OH)2)sulfuric acid (H2SO4)barium hydroxide (Ba(OH)2)2 more rows•May 8, 2021
What is the pH of kclo4?
to 7The pH of the aqueous solution has a pH equal to 7. So, the aqueous potassium perchlorate solution is neutral.
Is mgcl2 an acid or base?
0:001:53Is MgCl2 acidic, basic, or neutral (dissolved in water)? - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo we look at our rules we have this mgcl2. It's a salt of strong bases.MoreSo we look at our rules we have this mgcl2. It's a salt of strong bases.
Is HNO3 a base or acid?
HNO3 is a potent acid, a base, a nitrating agent and a heavy oxidising agent at times. In the presence of a stronger acid, it serves as a base. As the conjugate base is more stable, nitric acid is a stronger acid than nitrous acid.
Is kno2 a base or acid?
basicTherefore, neither ion will affect the acidity of the solution, so KCl is a neutral salt. Although the K + ion derives from a strong base (KOH), the NO 2 − ion derives from a weak acid (HNO 2). Therefore the solution will be basic, and KNO 2 is a basic salt.
Does KNO3 form a basic solution in water?
0:441:41Is KNO3 acidic, basic, or neutral (dissolved in water)? - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo if you have kno3. You put it in water it dissolves. You end up with a neutral. Solution.MoreSo if you have kno3. You put it in water it dissolves. You end up with a neutral. Solution.
Is HNO3 an acid?
Nitric acid (HNO₃) is a colorless liquid with yellow or red fumes with an acrid odor. Exposure to nitric acid can cause irritation to the eyes, skin, and mucous membrane; it can also cause delayed pulmonary edema, pneumonitis, bronchitis, and dental erosion. Nitric acid is highly corrosive.
What is the chemical formula for potassium chlorate?
Chemical compound. Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO 3. In its pure form, it is a white crystalline substance. It is the most common chlorate in industrial use.
What is molten potassium chlorate?
Molten potassium chlorate is an extremely powerful oxidizer and spontaneously reacts with many common materials such as sugar. Explosions have resulted from liquid chlorates spattering into the latex or PVC tubes of oxygen generators, as well as from contact between chlorates and hydrocarbon sealing greases.
What is the purpose of potassium chlorate cartridge?
Since 2005, a cartridge with potassium chlorate mixed with lactose and rosin is used for generating the white smoke signaling the election of new pope by a papal conclave . Potassium chlorate is often used in high school and college laboratories to generate oxygen gas.
How does potassium chlorate decompose?
Potassium chlorate readily decomposes if heated while in contact with a catalyst, typically manganese (IV) dioxide (MnO 2 ). Thus, it may be simply placed in a test tube and heated over a burner. If the test tube is equipped with a one-holed stopper and hose, warm oxygen can be drawn off. The reaction is as follows:
Can potassium chlorate ignite?
Mixtures of potassium chlorate and a fuel can ignite by contact with sulfuric acid, so it should be kept away from this reagent . Sulfur should be avoided in pyrotechnic compositions containing potassium chlorate, as these mixtures are prone to spontaneous deflagration.
What is Potassium Chlorate?
Potassium Chlorate is an inorganic compound with chemical formula KClO 3.
What temperature does potassium chlorate react with?
The thermal decomposition of potassium chlorate to obtain oxygen and potassium chloride. This reaction occurs at a temperature of between 150-300 ° C. For this reaction manganese (IV) oxide can be the catalyst.
Is potassium chlorate a crystalline substance?
It appears as a white crystalline substance in its pure form. It is the most widely used chlorate industry. The aqueous solution of potassium chlorate is a colourless liquid that is denser than water. It could be toxic when ingested. When it comes in contact it can irritate your eyes, skin, mucous membranes.
Is potassium chlorate thermal decomposition excessive?
Potassium chlorate thermal decomposition is not excessive, it’s just a redox reaction. Disproportionation refers to the same product that functions both as an oxidizing agent and as a reduction agent, resulting in compounds that contain the same product in different oxidation states.
Where is chlorate found?
A recent study has discovered the presence of natural chlorate deposits around the world, with relatively high concentrations found in arid and hyper-arid regions. The chlorate was also measured in rainfall samples with the amount of chlorate similar to perchlorate.
What is the formula for chlorate anion?
The chlorate anion has the formula ClO−. 3. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid.
What does "chlorate" mean in chemistry?
If a Roman numeral in brackets follows the word "chlorate", this indicates the oxyanion contains chlorine in the indicated oxidation state, namely: Using this convention, "chlorate" means any chlorine oxyanion. Commonly, "chlorate" refers only to chlorine in the +5 oxidation state.
How to make metal chlorates?
Metal chlorates can be prepared by adding chlorine to hot metal hydroxides like KOH :
Which anions have trigonal pyramidal structures?
As predicted by valence shell electron pair repulsion theory, chlorate anions have trigonal pyramidal structures .
Is chlorate a Lewis structure?
Structure and bonding. The chlorate ion cannot be satisfactorily represented by just one Lewis structure, since all the Cl–O bonds are the same length (1.49 Å in potassium chlorate ), and the chlorine atom is hypervalent. Instead, it is often thought of as a hybrid of multiple resonance structures :
Can chlorate be used in pyrotechnics?
Chlorates were once widely used in pyrotechnics for this reason, though their use has fallen due to their instability.