What kind of base is KOtBu?
Potassium tert-Butoxide (KOt-Bu) Is A Bulky Base Today's reagent, potassium tert-butoxide (KOt-Bu), is a strong base just like all alkoxides, but there's something about it that makes it special.
Is tert-butoxide a strong base?
Tert-butoxide (tert-butoxide ion; tBuO-): (CH3)3CO-; the conjugate base of tert-butanol. A strong base (frequently used in E2 and enolate reactions) but a fairly poor nucleophile due to steric hindrance.
Is NaOEt a strong base?
Sodium ethoxide, also referred to as sodium ethylate, is the organic compound with the formula C2H5ONa, or NaOEt. It is a white solid, although impure samples appear yellow or brown. It dissolves in polar solvents such as ethanol. It is commonly used as a strong base.
Is tert-butyl a strong base?
tert-Butyl alcohol (C4H10O) Its conjugate base, tert-butoxide is a strong base. Since it is bulky, tert-butoxide normally does not participate in nucleophilic substitution, such as in a Williamson ether synthesis or an SN2 reaction.
Why is T BuOK a strong base?
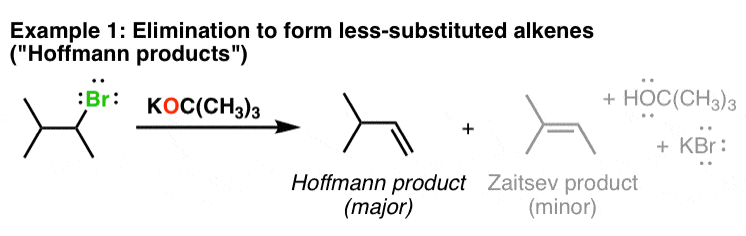
Several reasons: t-BuOK is especially known as a strong base, and a poor nucleophile. Its large, bulky structure causes it to perform exceptionally poorly in substitution, literally eliminating any side reactions when the desired product is the elimination product. It is easily available, like Raphaël insists.
Why tertiary butoxide is the strongest base?
In tert-butoxide three methyl groups push the electron density towards the oxygen atom through sigma bonds thereby enhance the electron density on oxygen atom, which is responsible for its higher basicity.
What are all of the strong bases?
List of Strong Bases (8):LiOH (lithium hydroxide)NaOH (sodium hydroxide)KOH (potassium hydroxide)Ca(OH)2 (calcium hydroxide)RbOH (rubidium hydroxide)Sr(OH)2 (strontium hydroxide)CsOH (cesium hydroxide)Ba(OH)2 (barium hydroxide)
Are Alkoxides strong bases?
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands.
Is NaOCH3 a weak base?
a. Since NaOCH3 is a strong nucleophile and base, it will force a 2nd-order mechanism. It is not a bulky base, so the 2° alkyl halide will give a mixture of E2 and SN2 products.
Is DBU a weak base?
DBU® and DBN are extremely strong basic organic compounds. It has excellent compatibility with various solvents and is useful as a catalyst for many organic synthesis reactions.
Is tert-Butyl alcohol acidic?
tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor....tert-Butyl alcohol.NamesAcidity (pKa)16.54Magnetic susceptibility (χ)5.742×10−5 cm3/molRefractive index (nD)1.387Dipole moment1.31 D60 more rows
How do you identify a strong base and a nucleophile?
If they bond to a hydrogen atom, we call them bases. If they bond to any other atom (especially carbon), we call them nucleophiles. A good base is usually a good nucleophile. So, strong bases — substances with negatively charged O, N, and C atoms — are strong nucleophiles.
Is NaNH2 a strong base?
NaNH2 is a strong base, intended to be strong enough to deprotonate the alkyne (pKa ≈ 25).
Is NaOCH2CH3 a strong base?
When you take an alkyl halide and add a strong base (such as NaOCH3 or NaOCH2CH3) a reaction occurs. See if you can recognize the bonds broken and formed.
Is CH3ONa a strong base?
CH3ONa is a strong base and a strong nu how name some main strong bases and - Chemistry - Alcohols Phenols and Ethers - 11760333 | Meritnation.com.
Is ch3li a strong base?
Methyllithium, CH3Li, is an incredibly strong base.